- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Ubituximab cuts relapse rate and extent of brain lesions among patients with recurrent MS: NEJM
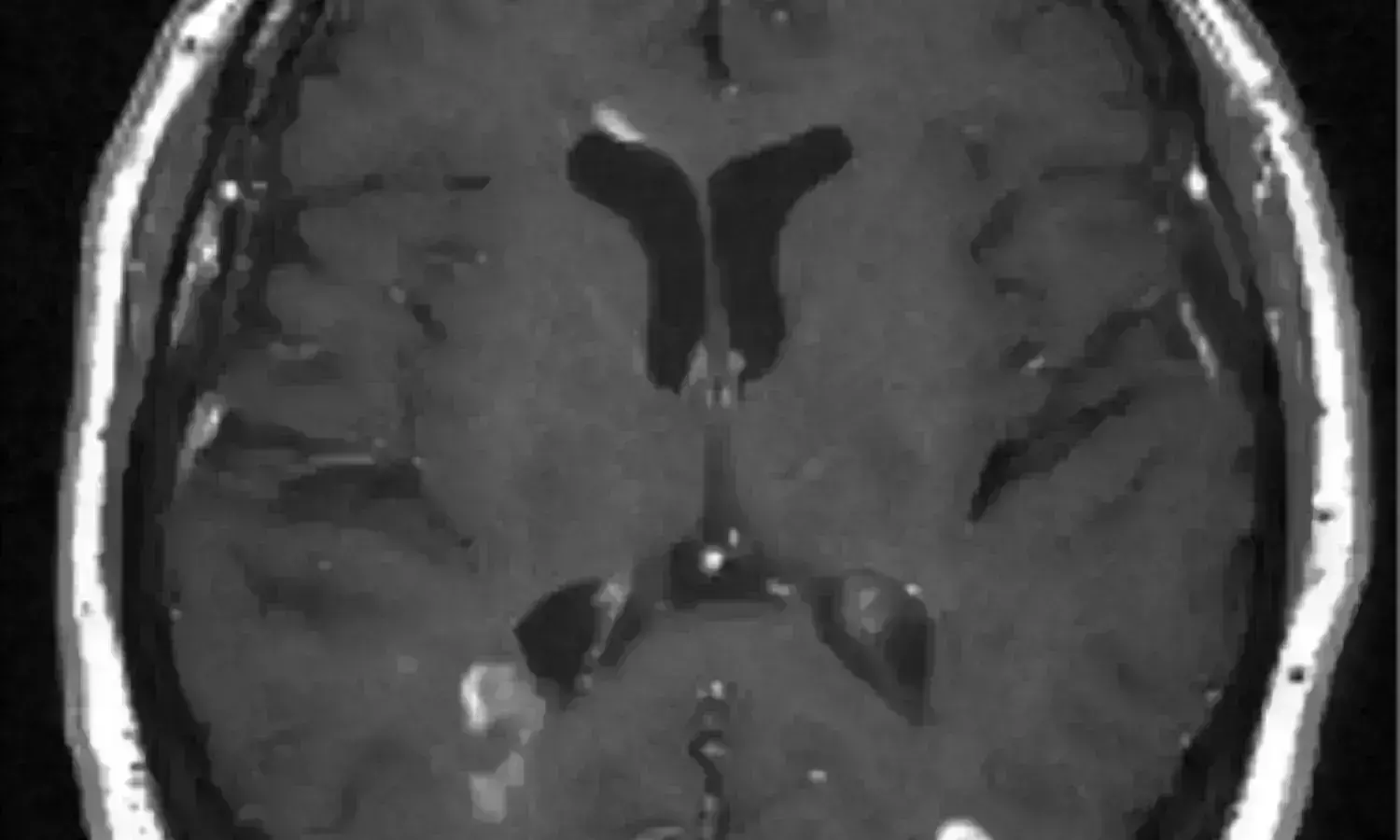
USA: When compared to teriflunomide, a frequently recommended oral treatment for multiple sclerosis, ubituximab, an experimental targeted B-cell therapy, exhibits greater efficacy, states a study published in The New England Journal of Medicine.
Typically developing between the ages of 20 and 40, MS is a neurological illness. It is brought on by an erroneous immune system response on the body's own myelin, which surrounds nerve fibers in the spine and brain and serves as a protective covering. Both the active or new brain lesions and the probability of relapse were significantly decreased in ULTIMATE I and II. Ublituximab marketing applications for treating adults with relapsing forms of multiple sclerosis (RMS) are now being examined by the US Food and Drug Administration and the European Medicines Agency.
"If authorized, ublituximab will be the first B-cell therapy available for patients with RMS that can be administered as a 1-hour infusion every six months after the initial dosage", added the authors.
For the research study, two identical, phase 3, double-blind, double-dummy trials were conducted with a total of 549 participants in the ULTIMATE I trial, and 545 in the ULTIMATE II trial. Relapsing multiple sclerosis patients were randomized in a 1:1 ratio to receive either intravenous teriflunomide (14 mg once daily) and oral placebo, or intravenous ublituximab (150 mg on day 1, followed by 450 mg on day 15 and at weeks 24, 48, and 72). The annual relapse rate served as the main outcome. The number of gadolinium-enhancing lesions on magnetic resonance imaging (MRI) after 96 weeks and a deterioration of functionality were considered secondary end goals.
Key findings of the study:
- In the ULTIMATE 1 trial, the mean number of gadolinium-enhancing lesions was 0.02 in the ublituximab group and 0.49 in the teriflunomide group (rate ratio [RR], 0.03; 95% CI, 0.02-0.06; P <.001); in the ULTIMATE 2 trial, the mean number was 0.01 and 0.25 (RR, 0.04; 95% CI, 0.02-0.06; < respectively.="" <="" p="">
- In terms of treatment safety, 89.2% of patients receiving ublituximab experienced at least 1 adverse event (AE), compared to 91.4% of patients receiving teriflunomide. In the ublituximab group, grade 3 or greater adverse events occurred slightly more frequently (21.3% vs 14.1%).
- In the predetermined pooled analysis, deterioration of disability was confirmed in 5.2% of those receiving ublituximab versus 5.9% of those receiving teriflunomide (hazard ratio [HR], 0.84; 95% CI, 0.50-1.41; P =.51).
- A worsening of impairment confirmed at 24 weeks was observed in 3.3% of patients receiving ublituximab compared to 4.8% of those receiving teriflunomide, but this difference was not deemed significant.
- In the ublituximab group, 47.7% of the individuals experienced infusion-related side effects. Serious infections affected 5.0% of those receiving ublituximab and 2.9% of people receiving teriflunomide.
The researchers concluded that over the course of 96 weeks, ublituximab was associated with lower annualized relapse rates and fewer brain lesions on MRI than teriflunomide, but it did not significantly reduce the chance of disability worsening.
REFERENCE
Steinman L, Fox E, Hartung HP, et al. Ublituximab versus teriflunomide in relapsing multiple sclerosis. N Engl J Med. 2022;387:704-714. doi:10.1056/NEJMoa2201904.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


