- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
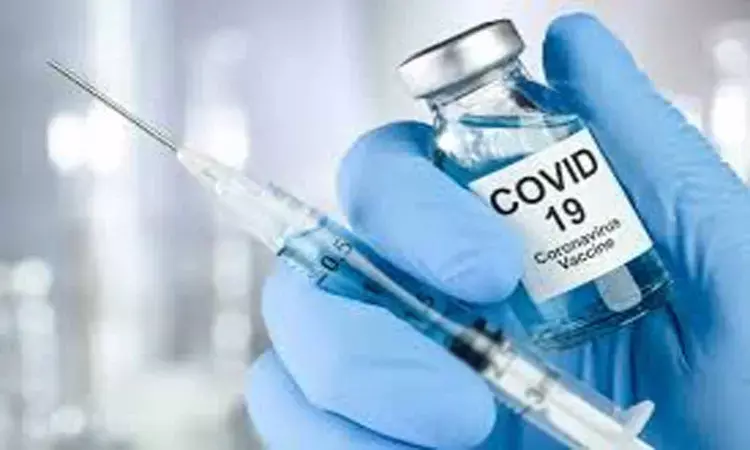
Bharat Biotech starts human trial of its anti-COVID vaccine at PGI Rohtak
“Three subjects were enrolled today. All have tolerated the vaccine very well. There were no adverse effects,” Vij further said in his tweet.
Chandigarh: The human trial of Bharat Biotech's anti-COVID-19 vaccine Covaxin began at Rohtak's Post-Graduate Institute of Medical Sciences on Friday, Haryana Health Minister Anil Vij tweeted.
In his tweet, the minister also said the vaccine has been administered to three persons who have shown no adverse effect to it.
"Human trial with Corona vaccine (COVAXIN) of Bharat Biotech started at PGI Rohtak today," said Vij, who is also the Home and Science & Technology minister.
"Three subjects were enrolled today. All have tolerated the vaccine very well. There were no adverse effects," Vij further said in his tweet.
The minister later told reporters in Ambala that "more vaccine candidates will be enrolled for the trial in coming days".
Bharat Biotech got the country's drug regulator's approval to start clinical trials of its anti-Corona vaccine Covaxin recently.
There are over seven anti-corona vaccines at various stages of development in the country with two of them having received the drug regulator's go-ahead to start the human clinical trials of their vaccines.
Earlier on Wednesday, Drug firm Zydus Cadila had said it has started human clinical trials of its COVID-19 vaccine candidate ZyCoV-D. In the phase of trials, the company will be enrolling over 1,000 subjects across multiple clinical study sites in India, it had said in a regulatory filing.
Zydus had earlier this month said it it has received approval from authorities to start human trials for its anti-COVID-19 vaccine.
Zydus had got the approval a few days after India''s first indigenous COVID-19 vaccine candidate COVAXIN, developed by Bharat Biotech in collaboration with Indian Council of Medical Research and National Institute of Virology, got the nod for human clinical trials from the Drug Controller General of India.
Read also: FIRST: Bharat Biotech Covid-19 vaccine COVAXIN gets DCGI nod for human trials
Ruchika Sharma joined Medical Dialogue as an Correspondent for the Business Section in 2019. She covers all the updates in the Pharmaceutical field, Policy, Insurance, Business Healthcare, Medical News, Health News, Pharma News, Healthcare and Investment. She has completed her B.Com from Delhi University and then pursued postgraduation in M.Com. She can be contacted at editorial@medicaldialogues.in Contact no. 011-43720751


