- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
CDSCO nod to ENTOD Pharma new eye drop to slow Myopia progression in children
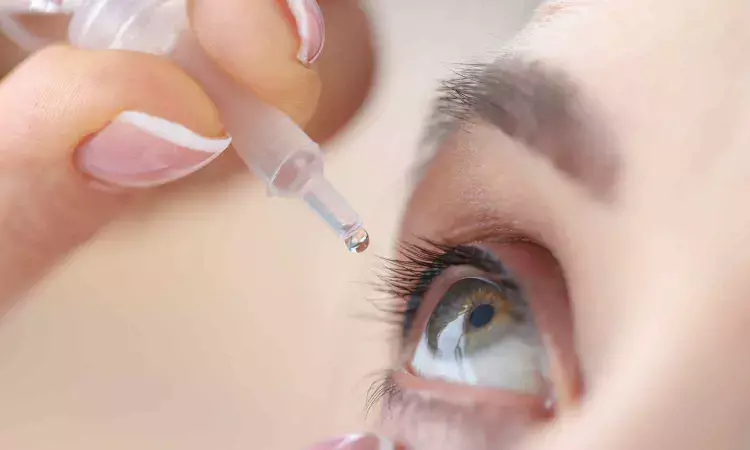
CDSCO granted marketing authorisation following the successful completion of a Phase 3 clinical trial conducted across 11 sites in India.
Mumbai: ENTOD Pharmaceuticals has received marketing authorisation from the Central Drugs Standard Control Organisation (CDSCO) for a new eye drop medicine developed to slow the progression of myopia in children aged 6 to 12 years.
"This historic milestone marks the first global regulatory approval for this unique treatment strength, and also highlights the growing impact & leadership of Indian innovation in the pharmaceutical sector The introduction of this pioneering therapy, developed and trialed by an Indian company, is poised to make a transformative impact on paediatric eye health in the country," the Company claimed.
The treatment will be available strictly by prescription from a registered medical practitioner for children diagnosed with myopia, following an evaluation by an eye doctor.
The Central Drugs Standard Control Organisation (CDSCO) granted marketing authorisation following the successful completion of a Phase 3 clinical trial conducted across 11 sites in India. The approval comes after a rigorous evaluation process by India’s apex drug regulator, further underscoring the robustness and credibility of the scientific data generated by ENTOD Pharmaceuticals’ research team.
Commenting on the CDSCO’s marketing approval, Mr. Nikkhil K Masurkar, CEO of ENTOD Pharmaceuticals, stated, "This is a proud moment for our research and regulatory teams. Securing regulatory approval for this unique treatment strength—the first of its kind globally—underscores our continued commitment to innovation in eye care. With myopia rates in India rising from 4% in 1999 to nearly 25% today, and projections suggesting that by 2050 one in two children could be affected, the need for such a therapy has never been more urgent.
Ruchika Sharma joined Medical Dialogue as an Correspondent for the Business Section in 2019. She covers all the updates in the Pharmaceutical field, Policy, Insurance, Business Healthcare, Medical News, Health News, Pharma News, Healthcare and Investment. She has completed her B.Com from Delhi University and then pursued postgraduation in M.Com. She can be contacted at editorial@medicaldialogues.in Contact no. 011-43720751
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


