- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
DCGI Moves to Enforce 2009 Ban on Single-Drug Artemisinin Pills Amid Resistance Concerns: Report
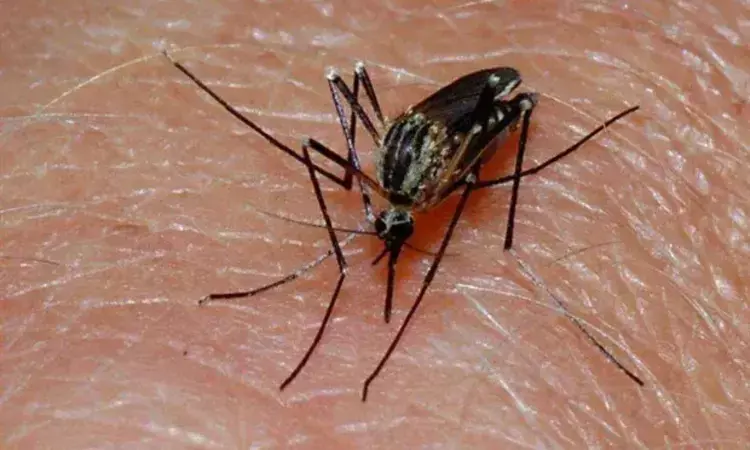
New Delhi: In a renewed push to safeguard India's malaria treatment protocols, the Drugs Controller General of India (DCGI) has directed all State and Union Territory drug regulators to identify, seize and halt the sale of banned single-drug oral artemisinin malaria pills, cautioning that their continued availability could accelerate the development of drug-resistant malaria strains.
The warning underscores the critical risk posed by monotherapies that undermine the global standard of combination-based malaria treatment.
According to a recent media report in LiveMint, DCGI Dr Rajeev Singh Raghuvanshi noted that despite a nationwide prohibition issued in 2009, certain manufacturers are still suspected of producing and distributing oral single-ingredient artemisinin formulations. These medicines, although capable of killing malaria parasites quickly, fail to remain in the bloodstream long enough to eliminate the infection completely, leaving behind stronger parasites that may lead to resistance.
International health guidelines have long discouraged the use of artemisinin monotherapy. The World Health Organization (WHO) advises that artemisinin must always be administered alongside another antimalarial to ensure full parasite clearance and prevent resistance from spreading. This resulted in the adoption of artemisinin-based combination therapies (ACTs) as the global gold standard. ACTs work through a two-step mechanism that reduces the likelihood of parasites surviving and developing resistance pathways.
Medical experts have warned that even isolated pockets of reduced drug sensitivity - particularly in some regions near India’s borders—could threaten the long-term success of ACTs. The DCGI’s directive reflects growing caution within the public health community that unregulated monotherapies, if allowed to circulate, could weaken the country’s malaria control strategies.
State drug authorities have been instructed to intensify surveillance, carry out targeted inspections, and remove any illegal single-drug artemisinin stocks found in the market. The manufacturing or sale of such products is a punishable offence under Section 26A of the Drugs and Cosmetics Act, 1940, inviting legal consequences including fines, imprisonment and license cancellation, reports LiveMint.
Industry observers note that while major pharmaceutical players dominate India’s $30-million antimalarial segment, officials did not disclose names of any companies suspected in the ongoing probe, stating that enforcement action remains underway.
M. Pharm (Pharmaceutics)
Parthika Patel has completed her Graduated B.Pharm from SSR COLLEGE OF PHARMACY and done M.Pharm in Pharmaceutics. She can be contacted at editorial@medicaldialogues.in. Contact no. 011-43720751


