- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
India finds multiple deficiencies among drugmakers following inspections across industry
Safipur CHC Burns Medicines, Investigation Launched
It also flagged an absence of raw materials testing, a lack of measures to avoid cross-contamination, an absence of professionally qualified employees, and faulty design of manufacturing and testing areas.
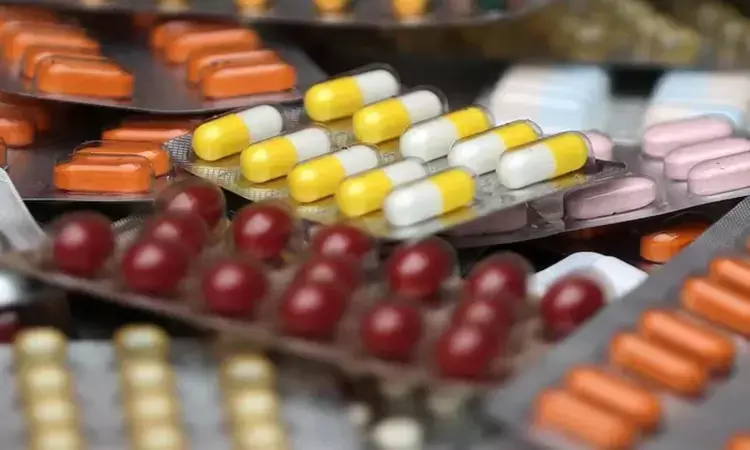
New Delhi: India has found multiple deficiencies among drugmakers following wide-ranging inspections across the industry, including a lack of raw materials testing, the health ministry said on Wednesday.
Indian authorities have stepped up scrutiny of drugmakers in recent months after some cough syrups made in the country were linked to the deaths of at least 95 children overseas.
Recent risk-based inspections of 162 factories and 14 public laboratories found issues including "poor documentation, lack of process and analytical validations, absence of self-assessment, absence of quality failure investigation, (and) absence of internal product quality review", it said in a statement.
It also flagged an absence of raw materials testing, a lack of measures to avoid cross-contamination, an absence of professionally qualified employees, and faulty design of manufacturing and testing areas.
India's $41 billion pharmaceutical industry is one of the biggest globally, known for providing cheaper alternatives to western products, but the recent cough syrup-related deaths have hurt that image.
The government has stopped production at four drugmakers so far after contaminants were flagged in their cough syrups by agencies including the World Health Organisation. The companies deny any wrongdoing.
The ministry said it has upgraded 'Good Manufacturing Practices' under the Drugs and Cosmetics Rules to check the deficiencies found during the inspections.
The upgrade includes the introduction of quality risk management, product quality review, supplier audit and approval, and validation of equipment.
Large drugmakers have been given six months and small manufacturers 12 months to transition to the upgraded manufacturing requirements, the ministry said.
India has tightened its testing of cough syrup exports since June, making it mandatory for companies to obtain a certificate of analysis from a government laboratory before exporting products.
cough syrupinspectionpharmaWorld Health OrganisationWHOGood Manufacturing PracticesDrugs and Cosmetics Rules
Source : ReutersRuchika Sharma joined Medical Dialogue as an Correspondent for the Business Section in 2019. She covers all the updates in the Pharmaceutical field, Policy, Insurance, Business Healthcare, Medical News, Health News, Pharma News, Healthcare and Investment. She has completed her B.Com from Delhi University and then pursued postgraduation in M.Com. She can be contacted at editorial@medicaldialogues.in Contact no. 011-43720751
Next Story


