- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Novo Nordisk Withdrawal to Reshape Insulin Market; Lupin's Huminsulin to Gain Spotlight
Mumbai: Amidst the ongoing news of conventional insulin market disruption and growing concern over potential shortages triggered by Novo Nordisk's decision to withdraw key human insulin products in India, Indian pharmaceutical companies are stepping in to stabilize supply, ensure accessibility and reassure patients.
Medical Dialogues had earlier reported that Novo Nordisk is phasing out several pen-fill and cartridge-based insulin formulations — including its popular brand Mixtard, as part of its global portfolio restructuring.
The move has spelt out a direct commercial impact for Abbott India, which has been marketing several of these insulin products in the country. With the pen-fill and cartridge formats being phased out, Abbott faces a narrowing of its insulin portfolio at a time when demand for convenient delivery systems remains high.
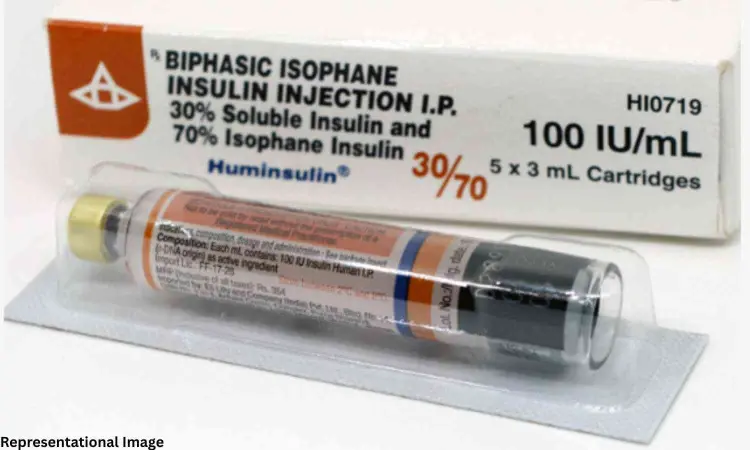
Amidst the fear of disruption in supply and accessibility, Indian pharmaceutical companies have stepped up to assure continuity of care. Speaking to the Medical Dialogues team, officials from Lupin, the second-largest player in India’s human insulin market* affirmed that their insulin brand — Huminsulin — remains fully available across formats.
Gagan Arora, Vice President – Lupin Metabolic, told Medical Dialogues, “At Lupin, patient access and uninterrupted supply are top priorities, especially for underserved.. Our insulin products are available pan-India in both vial and cartridge forms. Even if there are shifts in prescribing patterns due to recent market developments, we are well prepared to ensure seamless availability and any increase in demand. With the help of healthcare providers and the supply chain stakeholders across the country we will support all patients through this transition.”
According to IQVIA (MAT March 2025), Lupin’s Huminsulin is the second-largest human insulin brand in India showing Lupin’s consistent presence and growing acceptance among healthcare providers. The company had formally acquired Huminsulin from Eli Lilly and Company in December 2024, further consolidating its position in the diabetes care segment.
Premix insulins, such as biphasic human insulin (30/70), play an important role in managing blood glucose by addressing both fasting and postprandial spikes. Prefilled insulin pens, such as those available for Huminsulin, are generally considered more user-friendly, economical and may support better adherence, particularly among elderly patients or those initiated on insulin therapy.
*According to IQVIA (MAT March 2025),
Farhat Nasim joined Medical Dialogue an Editor for the Business Section in 2017. She Covers all the updates in the Pharmaceutical field, Policy, Insurance, Business Healthcare, Medical News, Health News, Pharma News, Healthcare and Investment. She is a graduate of St.Xavier’s College Ranchi. She can be contacted at editorial@medicaldialogues.in Contact no. 011-43720751


