- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Use of OdonAssist device tied to higher rate of successful assisted vaginal deliveries, finds study
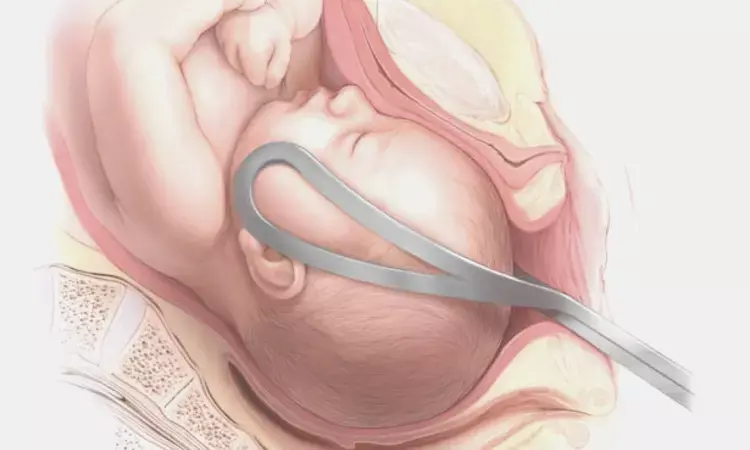
Recent research paper "Safety and efficacy of the OdonAssist inflatable device for assisted vaginal birth: the BESANCON ASSIST study" aimed to investigate the safety and efficacy of the OdonAssist inflatable device before conducting a randomized controlled trial. The study was an open-label, nonrandomized investigation of 104 women with clinically indicated assisted vaginal delivery using the OdonAssist at the Besancon University Hospital, France. The primary outcome measure was the proportion of successful assisted vaginal births using the OdonAssist, and the secondary outcomes included neonatal and maternal data, ease of use of the device, and maternal perception ratings of the birth experience.
Safety and Efficacy Results
The study reported a success rate of 88.5% for assisted vaginal births using the OdonAssist, with no emergency cesarean deliveries performed in the OdonAssist group, and no serious adverse maternal or neonatal reactions related to the use of the device. The rate of third- and fourth-degree perineal tears with the OdonAssist was 3.8%, and maternal perception ratings of the birth experience with the OdonAssist were high. The paper also highlighted the safety and efficacy of the OdonAssist as an alternative to other current devices for assisted vaginal births, with high acceptance rates among pregnant women and the high rate of successful assisted vaginal births using the OdonAssist confirming the feasibility of a future randomized controlled trial.
Design and Innovation
The study used an innovative design combining mechanical principles that favor the progression of the fetal head, including propulsion, flexion, and traction. The device was reported to be safer, easier to use, and more acceptable to women and obstetricians than currently available instruments. The success rate was higher than previously reported in a feasibility study, and the results confirmed the feasibility of conducting randomized controlled trials to evaluate the efficacy of OdonAssist in comparison with other assisted vaginal delivery devices.
Conclusion
Overall, the study concluded that the OdonAssist is a safe and effective alternative to other current devices for assisted vaginal births, with the high acceptance rate among pregnant women and the high rate of successful assisted vaginal births using the OdonAssist confirming the feasibility of a future randomized controlled trial. The detailed findings and safety and efficacy data from this study contribute to the existing knowledge on the OdonAssist device and support the feasibility of future randomized controlled trials to further evaluate its performance and safety in comparison with other assisted vaginal delivery devices.
Key Points
1. The study aimed to investigate the safety and efficacy of the OdonAssist inflatable device for assisted vaginal birth before conducting a randomized controlled trial.
2. Safety and efficacy results revealed a success rate of 88.5% for assisted vaginal births using the OdonAssist, with no emergency cesarean deliveries performed in the OdonAssist group and no serious adverse maternal or neonatal reactions related to the use of the device. The rate of third- and fourth-degree perineal tears with the OdonAssist was 3.8%, and maternal perception ratings of the birth experience with the OdonAssist were high.
3. The study used an innovative design combining mechanical principles favoring fetal head progression, including propulsion, flexion, and traction. The OdonAssist was reported to be safer, easier to use, and more acceptable to women and obstetricians than currently available instruments. The high success rate confirmed the feasibility of conducting randomized controlled trials to evaluate the efficacy of OdonAssist in comparison with other assisted vaginal delivery devices.
4. The OdonAssist was highlighted as a safe and effective alternative to other current devices for assisted vaginal births, with high acceptance rates among pregnant women. The detailed findings and safety and efficacy data from this study contribute to the existing knowledge on the OdonAssist device, supporting the feasibility of future randomized controlled trials to further evaluate its performance and safety in comparison with other assisted vaginal delivery devices.
5. The primary outcome measure was the proportion of successful assisted vaginal births using the OdonAssist, and the secondary outcomes included neonatal and maternal data, ease of use of the device, and maternal perception ratings of the birth experience.
6. Overall, the study concluded that the OdonAssist is a safe and effective alternative to other current devices for assisted vaginal births, with the high acceptance rate among pregnant women and the high rate of successful assisted vaginal births using the OdonAssist confirming the feasibility of a future randomized controlled trial.
Reference
Mottet N, Hotton E, Eckman-Lacroix A, Bourtembourg A, Metz JP, Cot S, Poitrey E, Delhomme L, Languerrand E, Nallet C, Lallemant M, Draycott T, Riethmuller D. Safety and efficacy of the OdonAssist inflatable device for assisted vaginal birth: the BESANCON ASSIST study. Am J Obstet Gynecol. 2024 Mar;230(3S):S947-S958. doi: 10.1016/j.ajog.2023.05.016. Epub 2023 Aug 1. PMID: 38462265.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


