- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Durvalumab plus Gemcitabine and Cisplatin in Advanced Biliary Tract Cancer: study
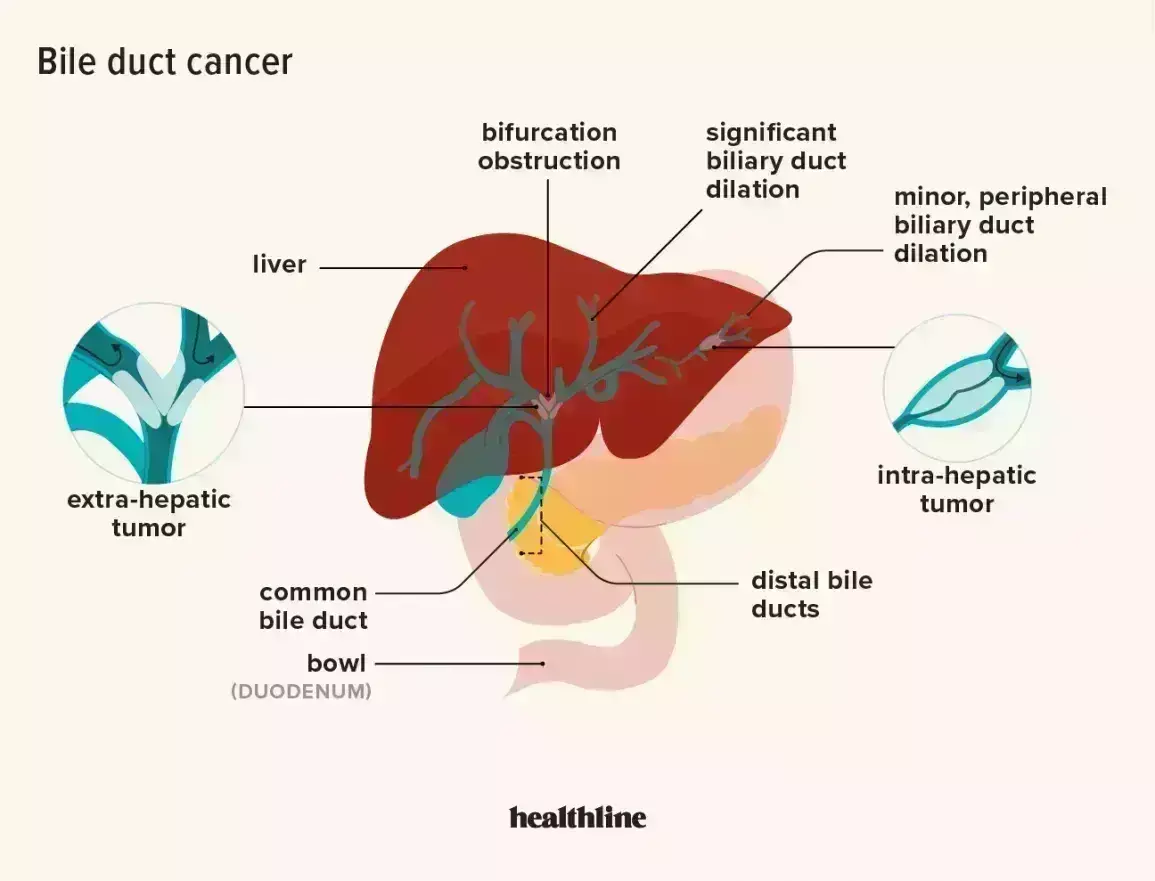
Durvalumab plus Gemcitabine and Cisplatin in Advanced Biliary Tract Cancer, according to a recent study published in the NEJM Evidence
Patients with advanced biliary tract cancer have a poor prognosis, and the first-line standard of care (gemcitabine plus cisplatin) has remained unchanged for more than 10 years. The TOPAZ-1 trial evaluated durvalumab plus chemotherapy for patients with advanced biliary tract cancer.
In this double-blind, placebo-controlled, phase 3 study, we randomly assigned patients with previously untreated unresectable or metastatic biliary tract cancer or with recurrent disease 1:1 to receive durvalumab or placebo in combination with gemcitabine plus cisplatin for up to eight cycles, followed by durvalumab or placebo monotherapy until disease progression or unacceptable toxicity. The primary objective was to assess overall survival. Secondary endpoints included progression-free survival, objective response rate, and safety.
Results:
- Overall, 685 patients were randomly assigned to durvalumab (n=341) or placebo (n=344) with chemotherapy. As of data cutoff, 198 patients (58.1%) in the durvalumab group and 226 patients (65.7%) in the placebo group had died.
- The hazard ratio for overall survival was 0.80 (95% confidence interval
- The estimated 24-month overall survival rate was 24.9% for durvalumab and 10.4% for placebo.
- The hazard ratio for progression-free survival was 0.75
- Objective response rates were 26.7% with durvalumab and 18.7% with placebo.
- The incidences of grade 3 or 4 adverse events were 75.7% and 77.8% with durvalumab and placebo, respectively.
Thus, Durvalumab plus chemotherapy significantly improved overall survival versus placebo plus chemotherapy and showed improvements versus placebo plus chemotherapy in prespecified secondary end points including progression-free survival and objective response rate. The safety profiles of the two treatment groups were similar.
Reference:
Durvalumab plus Gemcitabine and Cisplatin in Advanced Biliary Tract Cancer by Do-Youn Oh, et al. published in the NEJM Evidence
Keywords:
Durvalumab, Gemcitabine, Cisplatin, Advanced Biliary, Tract, Cancer, NEJM Evidence, Aiwu Ruth He, Shukui Qin, Li-Tzong Chen, Takuji Okusaka, Arndt Vogel, Jin Won Kim, Thatthan Suksombooncharoen, Myung Ah Lee, Masayuki Kitano,
Dr. Shravani Dali has completed her BDS from Pravara institute of medical sciences, loni. Following which she extensively worked in the healthcare sector for 2+ years. She has been actively involved in writing blogs in field of health and wellness. Currently she is pursuing her Masters of public health-health administration from Tata institute of social sciences. She can be contacted at editorial@medicaldialogues.in.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


