- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Researchers design gene therapy that can effectively target glioblastoma
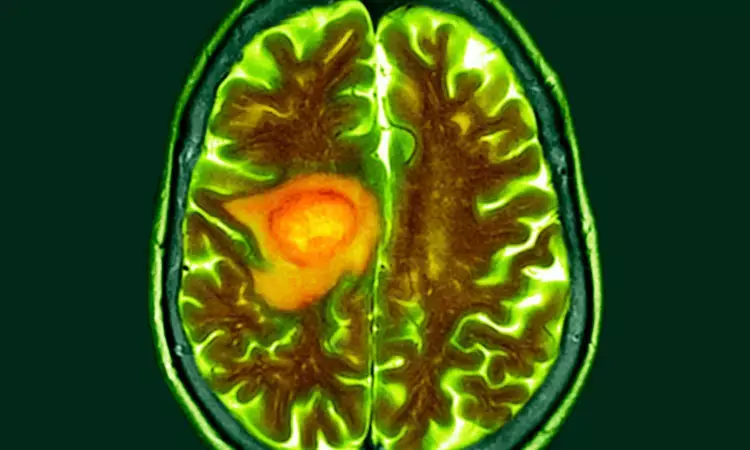
Glioblastoma (GBM), an aggressive brain cancer, is notoriously resistant to treatment, with recurrent GBM associated with survival of less than 10 months. Immunotherapies, which mobilize the body’s immune defenses against a cancer, have not been effective for GBM, in part because the tumor’s surrounding environment is largely impenetrable to assaults from the body’s immune system. To convert this immunosuppressive environment into one amenable to an immune response, investigators from Brigham and Women’s Hospital, a founding member of the Mass General Brigham healthcare system, engineered a novel oncolytic virus that can infect cancer cells and stimulate an anti-tumor immune response. Results, published in Nature, demonstrated the safety and preliminary efficacy of the novel gene therapy approach in high-grade glioma patients, with prolonged survival in a subgroup of recurrent GBM patients immunologically “familiar” with the virus.
“GBM has an aggressive effect in part because of a milieu of immunosuppressive factors surrounding the tumor, which enable the tumor’s growth by preventing the immune system from entering and attacking it,” said corresponding author E. Antonio Chiocca, MD, PhD, Chair of the BWH Department of Neurosurgery. “This study showed that with a virus we designed, we can reshape this ‘immune desert’ into a pro-inflammatory environment.”
This phase I, first-in-human trial examined the safety of an oncolytic virus, called CAN-3110, which was designed and subjected to preclinical testing by researchers at BWH and licensed to Candel Therapeutics as the trial was ongoing.
The cancer-attacking virus is an oncolytic herpes simplex virus (oHSV), which is the same type of virus used in a therapy approved for the treatment of metastatic melanoma. Unlike other clinical oHSVs, this therapy includes the ICP34.5 gene, which is often excluded from clinical oHSVs because it causes human disease in unmodified forms of the virus. However, the researchers hypothesized that this gene may be necessary to trigger a robust, pro-inflammatory response necessary for attacking the tumor. Therefore, they designed a version of the oHSV1 that contains the ICP34.5 gene but is also genetically “programmed” not to attack healthy brain cells.
Overall, the trial demonstrated the safety of CAN-3110 in 41 patients with high-grade gliomas, including 32 with recurrent GBM. The most serious adverse events were seizures in two participants. Notably, GBM participants who had pre-existing antibodies to HSV1 virus (66% of the patients) had a median overall survival of 14.2 months. In patients with pre-existing antibodies, the researchers saw markers of several changes in the tumor microenvironment associated with immune activation. They hypothesize that the presence of HSV1 antibodies resulted in a rapid immune response to the virus, which brought more immune cells to the tumor and increased the levels of inflammation in the tumor microenvironment.
After CAN-3110 treatment, the investigators also observed an increase in the diversity of the T cell repertoire, suggesting that the virus induces a broad immune response, perhaps by eliminating tumor cells resulting in the release of cancer antigens. These immunological changes after treatment were also shown to be associated with improved survival.
Studies like this one show the promise of gene therapy for treating intractable conditions. Mass General Brigham’s Gene and Cell Therapy Institute is helping to translate scientific discoveries made by researchers into first-in-human clinical trials and, ultimately, life-changing treatments for patients. The Institute’s multidisciplinary approach sets it apart from others in the space, helping researchers to rapidly advance new therapies and push the technological and clinical boundaries of this new frontier.
Going forward, the researchers plan to complete prospective studies to further investigate the effectiveness of the oncolytic virus in patients who do and do not have antibodies to HSV1. Having demonstrated the safety of one viral injection, they are proceeding to test the safety and efficacy of up to six injections over four months, which, like multiple rounds of vaccination, may increase the effectiveness of the therapy. The new, six-injection trial is funded by Break Through Cancer.
“Almost no immunotherapies for GBM have been able to increase immune infiltration to these tumors, but the virus studied here provoked a very reactive immune response with infiltration of tumor-killing T-cells,” Chiocca said. “That’s hard to do with GBM, so our findings are exciting and give us hope for our next steps.”
Reference:
Ling, A.L., Solomon, I.H., Landivar, A.M. et al. Clinical trial links oncolytic immunoactivation to survival in glioblastoma. Nature (2023). https://doi.org/10.1038/s41586-023-06623-2.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


