- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Vismodegib effective against advanced basal cell carcinoma, finds study
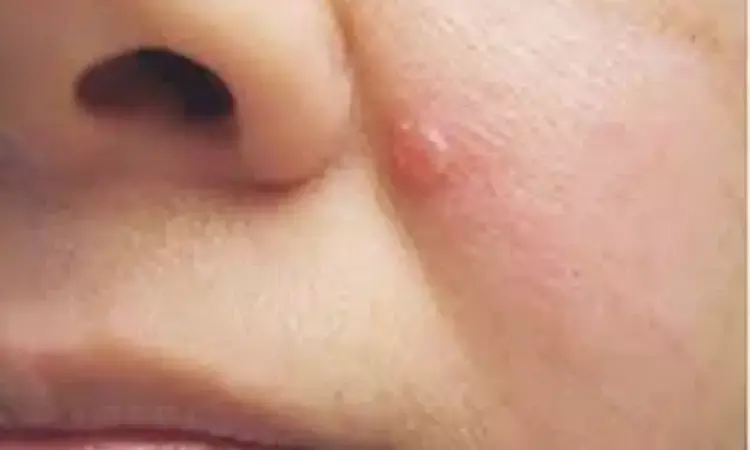
Canada: A recent study has reported vismodegib to be an effective treatment for advanced basal cell carcinoma (aBCC); however, toxicity remained an important factor to limit treatment duration. Also, a significant number of the treated patients became amenable to radiotherapy or surgery. The findings, published in the Journal of Cutaneous Medicine and Surgery, were based on real-world experience.
Vismodegib, a novel hedgehog pathway inhibitor, has revolutionized the treatment of aBCC patients who are poor candidates for surgery or radiation. However, not many studies have explored its use to facilitate further surgery or radiotherapy, and the optimal treatment duration to balance outcomes with adverse effects.
Justin Tong, Schulich School of Medicine & Dentistry, Western University, London, ON, Canada, and colleagues, therefore, performed a retrospective study to characterize the disease response, progression, and recurrence outcomes of BCC patients, and to report the impact of subsequent therapies.
The study included 46 adult patients with advanced basal cell carcinoma (aBCC), including both locally advanced (laBCC) and metastatic (mBCC) disease, treated with vismodegib at a single center from 2012 to 2019.
Thirty-six of them had laBCC, and 10 had mBCC. They were given treatment for over a mean of 21.9 months.
Following were the study's key findings:
- 50% had a complete response (CR), and 41.3% achieved partial response (PR).
- The median time to maximal response was 5.3 months.
- 23.9% had resected disease at median of 17.2 months, and 23.9% received radiotherapy.
- 69.6% experienced progressive disease after the achievement of CR or PR.
- Among 17 CR patients, who stopped treatment, 82.3% experienced subsequent relapse; 85% attained a repeat response.
- 43.5% discontinued treatment at least once due to adverse effects.
"With a response rate of 91%, London Regional Cancer Center's (LRCP)'s experience with vismodegib supports its effectiveness in treatment of aBCC," the authors wrote.
Reference:
1. Tong J, Mitchell B, Roth K, Logan D, Ernst S. Real-World Experience of Vismodegib in Advanced Basal Cell Carcinoma at a Canadian Cancer Center. Journal of Cutaneous Medicine and Surgery. October 2021. doi:10.1177/12034754211051234
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


