- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Cyclosporine 0.1% alternative to 2% dosage in Severe Vernal Keratoconjunctivitis in Children
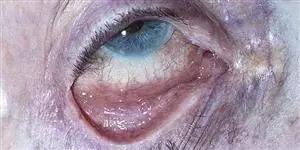 Courtesy British Journal of Ophthalmology
Courtesy British Journal of OphthalmologyVernal keratoconjunctivitis (VKC) is a severe and complex chronic inflammatory pathology affecting the ocular surface. It occurs in young children around 6 years of age and mainly affects boys. Usually seasonal and characterized by periods of exacerbation during hot, sunny periods, VKC generally progresses between the ages of 2 and 10 years. While the disease most often resolves by the end of puberty, 10% of cases become chronic and take the form of adult atopic keratoconjunctivitis. A history of atopic diseases is only found in 50% of cases.
Standardisation of the management of VKC is essential, particularly in order to limit the use of corticosteroids to severe flare-ups alone and prohibit them in stabilised forms. The most common iatrogenic complications associated with the long-term use of local corticosteroid treatment are infection of the ocular surface, development of glaucomatous optic neuropathy and cataract formation. The reduction of corticosteroid treatment is therefore a major element in the long-term management of patients with VKC.
In severe corticosteroid-dependent forms of VKC, maintenance treatment with a local immunomodulator is therefore indicated, with first-line introduction of a cyclosporine-based eye drop. The efficacy and safety of different concentrations of cyclosporine (0.5%, 1% and 2%) with regard to symptoms and reduced corticosteroid use have been demonstrated.
The aim of the study by Bourcier et al to compare the evolution in symptomatic and clinical scores, and need for topical corticosteroid treatment in a population of children with severe VKC treated with two dosages of cyclosporine treatment (0.1% and 2%). Data were compiled on inclusion then every three months from March, with a total follow-up duration of 12 months. Data concerning patient evolutions and complications were collected for the two treatment groups.
The mean age of the 46 children was 8.8 ±2.4 years with age at onset of symptoms of 5.1 ± 0.9 years. The cohort was predominantly (65%) male.
Corticosteroid dependence on inclusion was present in 52% of the children included. A significant improvement in the various symptomatic and clinical scores was observed following treatment with cyclosporine (0.1% and 2%).
Use of topical corticosteroid treatment reduced from 19 drops per month on inclusion to 4 drops per month at 12 months. Safety was comparable for the two groups.
Cyclosporine has proven its efficacy in managing severe VKC in children. An improvement in symptomatic and clinical scores was observed, regardless of cyclosporine posology. There was no difference in progression between the two concentrations. These progressions have also been observed previously for cyclosporine 2% and cyclosporine 0.1%. The early efficacy is primarily secondary to the effects of the topical corticosteroid treatment prescribed to all patients with severe VKC on inclusion.
The efficacy of cyclosporine from 15 days of use is mainly associated with inhibition of Th2 lymphocyte proliferation and IL-2 production, and a reduction in the number of cells and inflammatory mediators on the ocular surface. This study shows comparable efficacy for dosages of 2% and 0.1%.
Cyclosporine takes the form of a cationic emulsion attracted by the negatively charged particles of cell membranes, increasing its retention on the ocular surface. This could explain the similar efficacy despite a dose that is 20 times lower. In addition, the number of drops administered is also lower with cyclosporine 0.1% (approx. 2 drops per day on inclusion then 1 drop per day by end of follow-up) that with cyclosporine 2% (approx. 3 drops per day on inclusion then 2 drops per day by end of follow-up). The efficacy of a lower dose of cyclosporine could be useful for limiting the doses received by patients, but also as this enables the prescription of a drug that is more easily available thanks to an official formula which is easier to store after opening, for example during school hours.
It should be noted that clinical progression is identical regardless of the clinical form of VKC. In addition, visual acuity is maintained in both groups. Maintenance of visual acuity is data that is rarely compiled in studies of cyclosporine. This progression profile is reassuring and tends to show that complications are also of iatrogenic origin, in particular secondary to corticosteroid treatment. The reduction in corticosteroid use thus remains the key reason for introducing cyclosporine in severe VKC. This study has shown extensive use of corticosteroids with 19 drops per month of dexamethasone in the cyclosporine 0.1% group on inclusion and 18 in the cyclosporine 2% group. The number of drops per month fell to 4 drops per month in both groups after 12 months of follow-up.
The safety of the treatment was also evaluated using questionnaires. The most common adverse effects were a burning sensation on administration, tearing, redness, and transient blurred vision. Discomfort was experienced by 41% of children treated with cyclosporine 2% and 37% of those treated with cyclosporine 0.1% at three months. This rate of discomfort fell to 8% in both groups after one year of follow-up. Cyclosporine is thus relatively well tolerated. The reduction in concentration does not correlate with a reduction in discomfort.
In children with severe VKC, a significant reduction in symptomatic and clinical scores is observed with cyclosporine 2% and 0.1%. Both doses enabled a substantial reduction in the use of topical corticosteroids. The tolerance of both treatments is good and improves over the course of follow-up.
Source: Bourcier et al; Clinical Ophthalmology 2022:16
https://doi.org/10.2147/OPTH.S370414
Dr Ishan Kataria has done his MBBS from Medical College Bijapur and MS in Ophthalmology from Dr Vasant Rao Pawar Medical College, Nasik. Post completing MD, he pursuid Anterior Segment Fellowship from Sankara Eye Hospital and worked as a competent phaco and anterior segment consultant surgeon in a trust hospital in Bathinda for 2 years.He is currently pursuing Fellowship in Vitreo-Retina at Dr Sohan Singh Eye hospital Amritsar and is actively involved in various research activities under the guidance of the faculty.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


