- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
First-line bevacizumab treatment cost-effective for CRVO or HRVO-associated macular oedema: JAMA
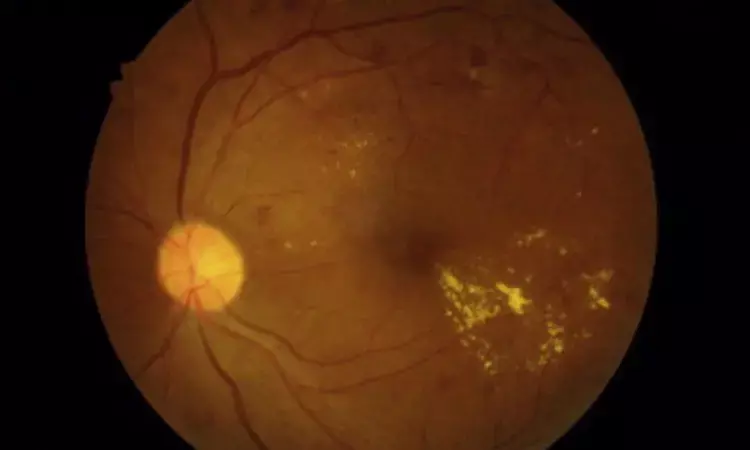
USA: First-line treatment with bevacizumab of macular oedema associated with central or hemiretinal vein occlusion was cost-effective in a new economic evaluation study published in JAMA Ophthalmology.
The study sought to answer the question of the relative cost-effectiveness of bevacizumab versus aflibercept for treating macular oedema associated with central (CRVO) or hemiretinal vein occlusion (HRVO). The findings were observed, given the minimal difference in visual acuity outcomes and significant cost differences between aflibercept and bevacizumab.
Retinal vein occlusion is the second most frequent retinal vascular disease. In the SCORE2 trial, bevacizumab was demonstrated to be noninferior to aflibercept concerning visual acuity in patients with macular oedema due to hemiretinal or central retinal vein occlusion after six months of therapy.
Steven M. Kymes, The Medwin Group, St Louis, Missouri, and colleagues aimed to investigate the relative cost-effectiveness of bevacizumab versus aflibercept for treating macular oedema associated with CRVO or HRVO.
The study used a microsimulation cohort of patients with demographic and clinical characteristics similar to SCORE2 participants and a Markov process. The simulated cohort comprised 5000 patients assessed 100 times, each with a different characteristic set randomly selected based on the SCORE2 trial. The parameters were estimated and validated using a split-sample approach of the SCORE2 population.
The patients were randomized to either Bevacizumab (followed by aflibercept in patients with a protocol-defined poor or marginal response to bevacizumab at six months) versus aflibercept (followed by a dexamethasone implant in patients with a protocol-defined poor or marginal response to aflibercept at six months). The incremental cost-utility ratio was the study's primary outcome.
The study led to the following findings:
- The simulation showed that patients treated with aflibercept would have an expected cost of $18 127 more remarkable than those treated with bevacizumab the year after the initiation.
- When coupled with the lack of clinical superiority over bevacizumab (i.e., patients with bevacizumab treatment had a gain over aflibercept in visual acuity letter score of 4 in the treated eye and 2 in the fellow eye), these findings demonstrate that the first-line treatment with bevacizumab dominated aflibercept in the simulated cohort of SCORE2 participants.
- At current price levels, aflibercept would be considered the preferred cost-effective option only if treatment restored the patient to nearly perfect health.
"While there will be some patients with macular oedema associated with CRVO or HRVO who will benefit from first-line treatment with aflibercept compared to bevacizumab," the researchers wrote. Given the minimal differences in visual acuity outcomes and significant cost differences for bevacizumab vs aflibercept, "first-line treatment with bevacizumab is cost-effective for this condition," they concluded.
Reference:
Kymes SM, Oden NL, VanVeldhuisen PC, et al. Cost-Utility Comparison of Bevacizumab and Aflibercept in the Treatment of Central or Hemiretinal Vein Occlusion in the SCORE2 Trial. JAMA Ophthalmol. Published online May 11, 2023. doi:10.1001/jamaophthalmol.2023.1463
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


