- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Oxymetazoline eye drops, a promising treatment for ptosis: JAMA
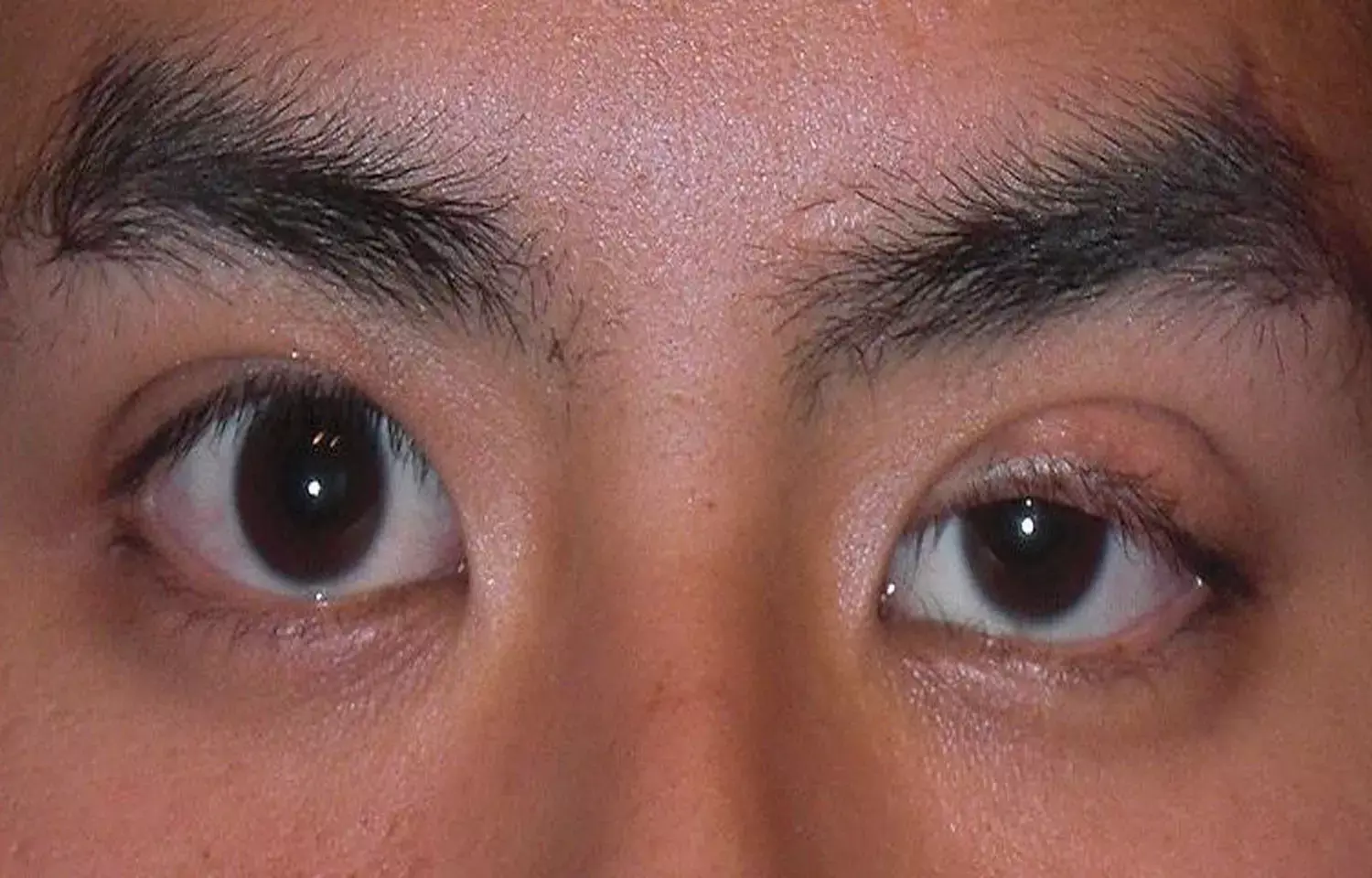
USA: Oxymetazoline, 0.1% may be a promising treatment for acquired ptosis, suggest a recent study in the journal JAMA Ophthalmology. This will do away with the need to undergo surgery for treating the condition.
Blepharoptosis (or ptosis) is drooping down of the upper eyelid margin with the eye in primary gaze. The eyelid disorder is broadly categorized as either congenital or acquired. Currently, the treatment of acquired blepharoptosis (ptosis) is currently limited to surgical intervention. Charles B. Slonim, University of South Florida Morsani College of Medicine, Tampa, and colleagues examined the efficacy and safety of oxymetazoline hydrochloride, 0.1%, ophthalmic solution (oxymetazoline, 0.1%) in participants with acquired ptosis.
It included participants 9 years and older with acquired ptosis and superior visual field deficit. The 2 studies were conducted across 16 and 27 sites in the United States.
The study enrolled 304 participants (mean age 63.8 years; 73% were women) from May 2015 to April 2019. Overall, 97.5% of participants received oxymetazoline, 0.1%, and 97.0% (98 of 101) of participants receiving the vehicle completed the studies. Participants were randomized in the ratio 2:1 to receive oxymetazoline, 0.1%, or vehicle, self-administered as a single drop per eye, once daily, for 42 days.
Key findings of the study include:
- Oxymetazoline, 0.1%, was associated with a significant increase in the mean (SD) number of points seen on the Leicester Peripheral Field Test vs vehicle (day 1: 5.9 vs 1.8; day 14: 7.1 vs 2.4).
- Oxymetazoline, 0.1%, also was associated with a significant increase in marginal reflex distance 1 from baseline (mean [SD]: day 1: 0.96 mm vs 0.50 mm; day 14: 1.16 mm vs 0.50 mm).
- Treatment-emergent adverse events (TEAEs) occurred in 31.0% of participants receiving oxymetazoline, 0.1%, and 35.6% of participants receiving vehicle.
- Among participants receiving oxymetazoline, 0.1%, with a TEAE, 81%n had a maximum TEAE intensity of mild, and 62% had no TEAE suspected of being treatment-related.
"Oxymetazoline, 0.1%, was associated with positive outcomes and was well tolerated in phase 3 trials after instillation at days 1 and 14, demonstrating its potential promise for the treatment of acquired ptosis. Further study is needed to elucidate the clinical relevance of these findings beyond 6 weeks," concluded the authors.
The study, "Association of Oxymetazoline Hydrochloride, 0.1%, Solution Administration With Visual Field in Acquired Ptosis: A Pooled Analysis of 2 Randomized Clinical Trials," is published in the journal JAMA Ophthalmology.
DOI: https://jamanetwork.com/journals/jamaophthalmology/fullarticle/2771169
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


