- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
FDA grants expanded approval to OTC pain device
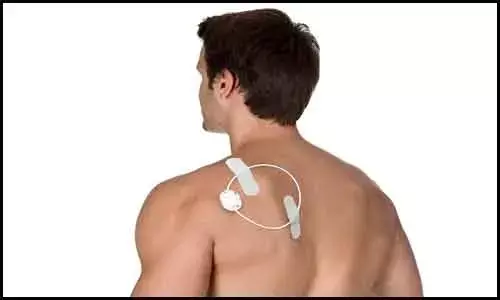
ActiPatch — an over-the-counter electromagnetic neuromodulation device for treating pain, manufactured by BioElectronics Corp has been granted US Food and Drug Administration (FDA) approval for marketing.
The new 510(k) clearance is indicated for adjunctive treatment of any musculoskeletal pain. It is clinically proven to reduce pain, increase the overall quality of life and reduce the number of painkillers taken.
The ActiPatch was already FDA-cleared for over-the-counter treatment of pain from knee osteoarthritis and plantar fasciitis (heel pain) in 2017. The latest clearance expands these indications to cover all musculoskeletal pain complaints. Keith Nalepka, Vice President Sales and Marketing, expressed confidence on exploiting the expanded market opportunity: "The expanded clearance paves the way for new products to be marketed with approved medical claims for musculoskeletal pain, for instance, in the back, knee, hips, wrists, elbow, and ankle."
ActiPatch works on this faulty signalling: When nerves in painful knee constantly send pain signals over a long period of time, the nerves "learn" to "over-react", like developing an allergy. During activities like walking or bending, instead of communicating normally with your brain, the nerves in your spine "over-react" and falsely send pain signals to the brain—and deliver "real" pain signals much more strongly than they normally would.
Kelly Whelan, President of BioElectronics, stated: "The Company intends to capitalize on this new clearance by offering additional products to retail channel partners, in alignment with our 2020 strategy to prioritize the OEM (Original Equipment Manufacturer) aspects of our business." With the latest clearance, ActiPatch remains the only pulsed shortwave therapy (PSWT) device with an over-the-counter clearance for treating any form of musculoskeletal pain."
The 510(k) application was prepared by the R&D team comprising: Kenneth McLeod, Ph.D., Director of Clinical Science and Engineering Research, State University of New York at Binghamton and Richard Staelin, Ph.D., Gregory Mario and Jeremy Mario Professors, Duke University, Ian Rawe, Ph.D., Director of Clinical Research, BioElectronics and Sree Koneru, Ph.D., VP Product Development, BioElectronics.
For more information, please visit www.tryactipatch.com
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


