- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Injectable drug for faster healing of bone fractures ready for trials
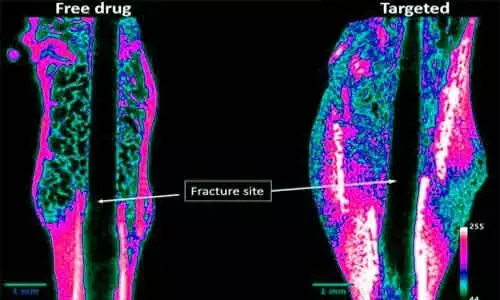
WEST LAFAYETTE - One in three adults aged 60 and over suffering from a hip fracture dies within one year. Healing of fracture in old age is a serious problem.
Now, a Purdue University-affiliated startup is moving closer to the start of clinical trials for a novel injectable drug that is targeted to heal broken bones faster and strengthen weak bones. The Purdue University discovered drug, NOV004, from Novosteo Inc., is unique in that it concentrates at the fracture site while reducing exposure to the rest of the body.
Novosteo, the startup developing the drug, was co-founded by father-son team Philip S. Low, the Presidential Scholar for Drug Discovery and the Ralph C. Corley Distinguished Professor of Chemistry, and Stewart A. Low, the company's CSO and Visiting Scholar in Purdue's Department of Chemistry. The treatment was developed in the Purdue laboratory of Low in the Purdue Institute of Drug Discovery. Currently, there are 288 clinical trials performed or in the process using Purdue-developed medical treatments at 4,841 sites across the globe.
Stewart A. Low said bone fractures can pose several risks to patients.
"People over 65 years of age who experience bone fractures, specifically hip fractures, have a one in four chance of dying from fracture-related complications. Half of these patients will not regain full mobility within a year," he said. "We plan to focus initially on hip fractures in the elderly. We believe this is an area of underdevelopment and concern, so our goal is to help provide a better solution for these patients."
Novosteo is developing fracture-targeted bone anabolic agents that selectively accumulate on the bone fracture surface where they accelerate the healing process.
Scott Salka, who recently joined the startup as executive chair, will use his 28 years of experience as a biotechnology entrepreneur to help Novosteo move its innovations from the laboratory to clinical trials and ultimately into the hands of doctors and patients.
"We have been working on some amazing science with people truly dedicated to making a difference in reducing the mortality and improving the quality of life for our ageing population," said Salka. "We have completed preclinical studies with NOV004 and are looking to take it to clinical trials later this year."
As CEO, Salka has successfully led efforts to advance novel drugs through preclinical and early clinical development, most recently at publicly traded Ampliphi, now Armata NYSE: ARMP. Prior to that he founded and served as CEO for both Ambit Biosciences, acquired by Daiichi Sankyo OTC: DSNKY, and Rakuten Medical.
Novosteo is already looking at the future use of the injectable-targeted drug for other applications, including dental implants, head and facial fractures, and hip and knee replacements. In addition, Novosteo has a pipeline of drugs for treating an array of musculoskeletal maladies. Salka will present some of the technology at Biocom's Global Life Science Partnering Conference this month in La Jolla, California.
Novosteo's technology is licensed through the Purdue Research Foundation Office of Technology Commercialization. The company also received entrepreneurial support from Purdue Foundry, an entrepreneurship and commercialization hub in Discovery Park District's Convergence Center for Innovation and Collaboration where startups, entrepreneurs, innovators, and companies can collaborate with Purdue to address global challenges in health, sustainability, IT and space.
The Purdue Institute of Drug Discovery is situated near the district, a $1 billion-plus long-term enterprise to support a transformational center of innovation on the western edge of the Purdue University campus. The district already includes a public airport with a 7,000-foot runway, and partnerships international companies including Rolls-Royce, Schweitzer Engineering Laboratories and Saab. Visit Discovery Park District.
for further references log on to:
https://www.novosteo.com/?_ga=2.115219983.961334498.1581489793-1329997001.1581489793
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


