- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Firsekibart Superior to Colchicine for Preventing Acute Gout Flares: Study
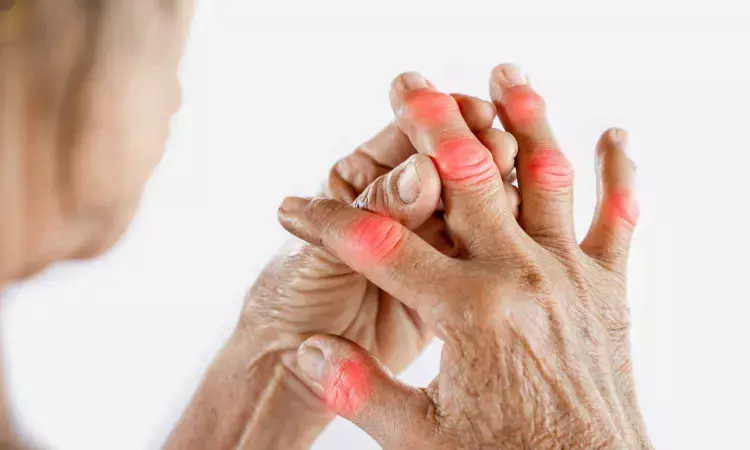
A new study published in the journal of ACR Open Rheumatology showed that firsekibart surpassed colchicine in reducing acute gout flares among individuals initiating urate-lowering treatment.
Firsekibart is emerging as a viable alternative for avoiding acute gout flares in persons initiating urate-lowering medication (ULT), a period when flare risk normally increases owing to changing serum urate levels. As ULT mobilizes urate deposits, patients typically endure severe episodes despite long-term advantages. Firsekibart, a targeted anti-inflammatory drug, is aimed to smooth this transition by lowering early flare incidence and enhancing therapy tolerance.
By delivering a preventive strategy, it may help patients adhere more comfortably to ULT, eventually enabling improved long-term gout management. According to preliminary clinical results, firsekibart has a good safety profile and can successfully reduce flare frequency, which makes it a viable supplement to existing gout management techniques during ULT commencement. Therefore, this trial investigated the effectiveness and safety of firsekibart vs colchicine for prophylaxis against acute gout flares in patients commencing ULT.
The participants in this study were randomly assigned (1:1:1) to receive either oral colchicine 0.5 mg/day for 12 weeks or a single subcutaneous injection of firsekibart 100 mg or 200 mg. ULT was begun at baseline or within 1 week before screening. The main objective was the mean number of acute gout flares per participant across 12 weeks.
A total of 162 individuals were recruited and allocated to receive firsekibart 100 mg (n = 55), firsekibart 200 mg (n = 52), or colchicine (n = 55). All groups had comparable baseline characteristics. Notably, the 200 mg firsekibart group had no flares for the first 12 weeks.
The adjusted mean number of acute gout flares per individual was 0.02 with firsekibart 100 mg versus 0.34 with colchicine, resulting with a rate ratio of 0.05 (95% CI 0.01-0.43; P = 0.0060). Across both firsekibart dosages, fewer people reported at least one flare when compared to the colchicine group. Firsekibart also showed good safety, with no adverse events that required therapy termination or trial withdrawal, no treatment-related severe events, and no fatalities.
Overall, gout is a widely frequent illness with an increasing worldwide burden and is typically linked with major comorbidities. The findings in this study imply that firsekibart may serve as a unique method for reducing acute gout flares in patients commencing ULT, possibly altering the therapy paradigm for gout.
Reference:
Yu, Y., Xue, Y., Hu, J., Zhang, N., Li, R., Guo, H., Qian, L., Gou, W., Yang, J., Mao, L., Yu, J., Liu, S., Xu, Q., Luo, T., & Zou, H. (2025). Firsekibart as a prophylactic treatment for acute gout flare in participants initiating urate-lowering therapy: A phase 2, randomized, open-label, multicenter, active-controlled trial. ACR Open Rheumatology, 7(11), e70111. https://doi.org/10.1002/acr2.70111
Neuroscience Masters graduate
Jacinthlyn Sylvia, a Neuroscience Master's graduate from Chennai has worked extensively in deciphering the neurobiology of cognition and motor control in aging. She also has spread-out exposure to Neurosurgery from her Bachelor’s. She is currently involved in active Neuro-Oncology research. She is an upcoming neuroscientist with a fiery passion for writing. Her news cover at Medical Dialogues feature recent discoveries and updates from the healthcare and biomedical research fields. She can be reached at editorial@medicaldialogues.in
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


