- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Bronchial thermoplasty found safe, effective and durable treatment for severe asthma
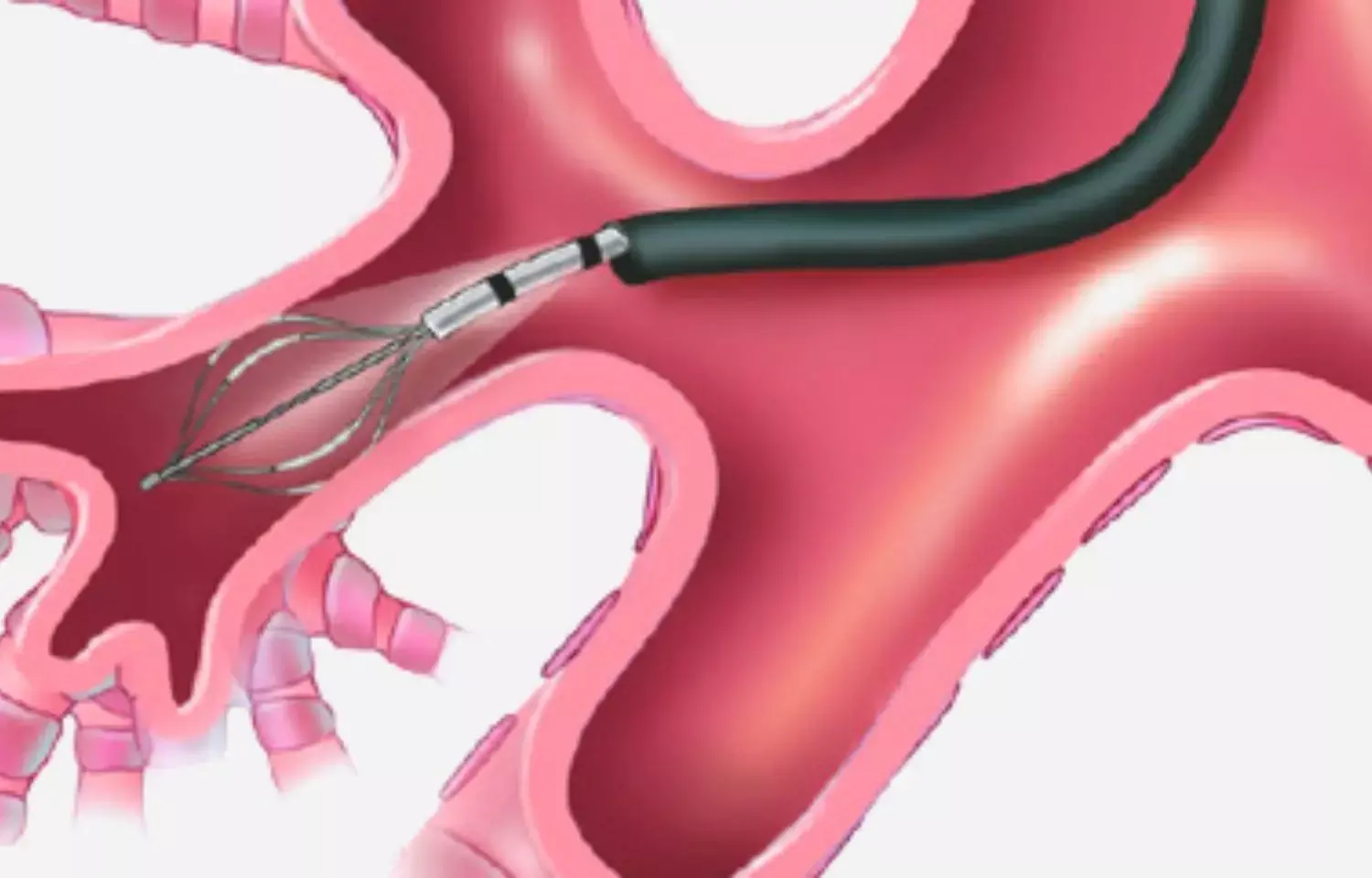
USA: Five years after treatment with bronchial thermoplasty patients with severe asthma experienced a decrease in exacerbations, ED visits, hospitalization, and corticosteroid exposure, researchers report in a new study.
Bronchial thermoplasty is a device-based treatment used for people ≥ 18 years of age with severe asthma poorly controlled with inhaled corticosteroids and long-acting beta-agonists. In the Post-FDA Approval Clinical Trial Evaluating Bronchial Thermoplasty in Severe Persistent Asthma (PAS2) study, data collection in patients with severe asthma undergoing this procedure was done.
In the study published in the Chest Journal, Geoffrey Chupp, Yale University, New Haven, CT, and colleagues address the question of the 5-year efficacy and safety results in patients with severe asthma who have undergone bronchial thermoplasty.
The study was a prospective, open-label, observational, multicenter study conducted in the United States and Canada. People included were of 18 to 65 years of age who were taking inhaled corticosteroids ≥ 1,000 μg/d (beclomethasone or equivalent) and long-acting beta-agonists ≥ 80 μg/d (salmeterol or equivalent). The researchers evaluated hospitalization, severe exacerbations, medication usage, and ED visits for the 12 months prior to and at years 1 through 5 posttreatment. Also, spirometry was evaluated at baseline and at years 1 through 5 posttreatment.
The research led to the following findings:
- A total of 284 subjects were enrolled at 27 centers; 227 subjects (80%) completed 5 years of follow-up. By year 5 posttreatment, the proportion of subjects with severe exacerbations, ED visits, and hospitalizations was 42.7%, 7.9%, and 4.8%, respectively, compared with 77.8%, 29.4%, and 16.1% in the 12 months prior to treatment.
- The proportion of subjects on maintenance oral corticosteroids decreased from 19.4% at baseline to 9.7% at 5 years.
- Analyses of subgroups based on baseline clinical and biomarker characteristics revealed a statistically significant clinical improvement among all subgroups.
"We found that five years following the treatment, subjects experienced decreases in severe exacerbations, ED visits, hospitalizations, and corticosteroid exposure," wrote the authors. "All subgroups showed clinically significant improvement, suggesting that the treatment (bronchial thermoplasty) improves asthma control in difference asthma phenotypes."
Reference:
The study titled, "Bronchial Thermoplasty in Patients With Severe Asthma at 5 Years: The Post-FDA Approval Clinical Trial Evaluating Bronchial Thermoplasty in Severe Persistent Asthma Study," was published in the Chest journal.
DOI: https://doi.org/10.1016/j.chest.2021.10.044
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


