- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Pirfenidone found safe, efficacious in idiopathic pulmonary fibrosis
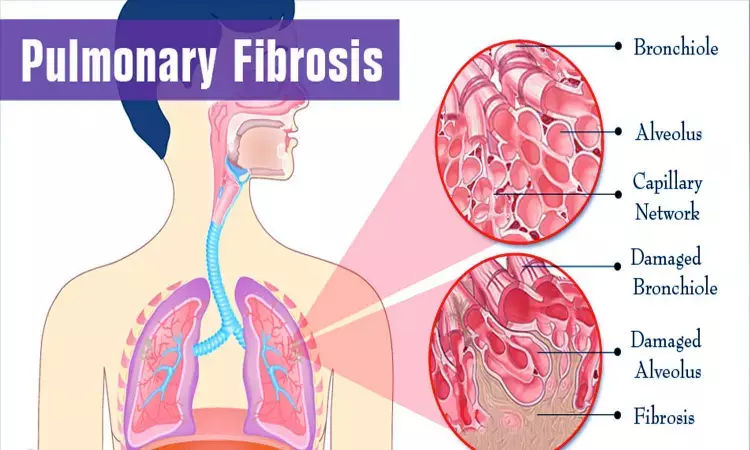
Pirfenidone has been found safe, and efficacious in idiopathic pulmonary fibrosis (IPF) in a real-world cohort study.
Pirfenidone is an antifibrotic agent approved for the treatment of idiopathic pulmonary fibrosis (IPF). The PolExPIR study aimed to describe the real-world data (RWD) on the Polish experience of pirfenidone therapy in IPF with respect to safety and efficacy profiles.
Researchers have found in a retrospective, observational study that Pirfenidone is safe and effective in idiopathic pulmonary fibrosis (IPF) in a real-world setting.
The PolExPIR study for ascertaining the effectiveness of Pirfenidone in idiopathic pulmonary fibrosis (IPF) is the first real-world cohort study although 16.6% of patients dropped out of it because of adverse drug reactions, and dose-adjustment rates were high. The findings of research have been published in BMC Pulmonary Medicine.
The researchers conducted a retrospective, multicenter study in Poland of 307 patients with IPF, covering 2017-2019.
The researchers identified a total of 307 patients receiving pirfenidone for analysis. The mean age was 68.83 (8.13) years and 77% were males. The median time from the first symptoms to IPF diagnosis was 15.5 (9.75-30) months and from diagnosis to start of pirfenidone treatment was 6 (2-23) months. Patients were followed on treatment for a median of 17 (12-22.75) months. The
Mean age was 68.83 (standard deviation, ±8.13) years and 77% of subjects were males.
The median time from first symptoms to IPF diagnosis was15.5 (interquartile range [IQR], 9.75-30) months.
The Patients were followed on pirfenidone for a median of 17 (IQR, 12-22.75) months.
In all 24.1% of patients needed dose adjustments and 11.4% were treated with a dose different from the full recommended dose.
A total of45.92% of patients discontinued therapy because of:
Adverse drug reactions (16.61%, most of them gastrointestinal or skin-related).
Disease progression (6.51%).
Patient request (5.54%).
Neoplastic disease (3.91%).
Lung transplantation (0.33%).
The Researchers found that Pulmonary function remained largely stable during 24 months of follow-up:
Median (IQR) annual decline in forced vital capacity:
First year: −20 (−200 to 100) mL.
Second year: −120 (−340 to 30) mL.
10.75% of patients died.
The researchers concluded that
the PolExPIR study was a source of longitudinal RWD on pirfenidone therapy in the Polish cohort of patients with IPF supporting its long-term acceptable safety and efficacy profiles and reinforce findings from the previous randomised clinical trials and observational studies.
For further Reference log on to:
Majewski S, Białas AJ, Buchczyk M, Gomółka P, Górska K, Jagielska-Len H, Jarzemska A, Jassem E, Jastrzębski D, Kania A, Koprowski M, Krenke R, Kuś J, Lewandowska K, Martusewicz-Boros MM, Roszkowski-Śliż K, Siemińska A, Sładek K, Sobiecka M, Szewczyk K, Tomczak M, Tomkowski W, Wiatr E, Ziora D, Żołnowska B, Piotrowski WJ. A multicentre retrospective observational study on Polish experience of pirfenidone therapy in patients with idiopathic pulmonary fibrosis: the PolExPIR study. BMC Pulm Med. 2020;20(1):122. doi: 10.1186/s12890-020-1162-6. PMID: 32366291
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


