- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Sultiame Once Daily Cuts Sleep Apnoea Events by Up to 35 Percent, Lancet Study Finds
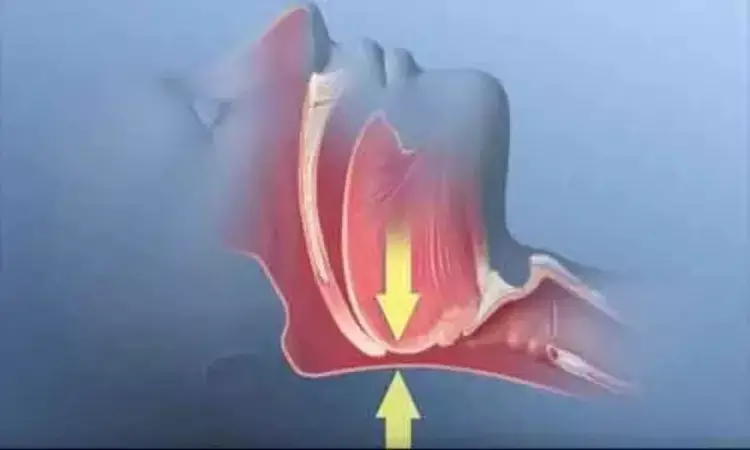
Germany: A new multicentre phase 2 clinical trial published in The Lancet has reported encouraging results for sultiame-a long-used anticonvulsant-as a potential once-daily treatment option for obstructive sleep apnoea (OSA).
Led by Prof Winfried Randerath from the University of Cologne and Bethanien Hospital, Solingen, Germany, the study found that the medication significantly reduced breathing disturbances and improved sleep-related symptoms over 15 weeks.
OSA affects a substantial portion of the global population, with roughly 15% of men and 7% of women living with the condition. Despite its prevalence, approved pharmacological treatments remain unavailable. Continuous positive airway pressure (CPAP) therapy is currently the gold standard, but long-term adherence is typically low, driving the search for alternative therapies. Sultiame, although widely used in Europe for epilepsy, has not been approved for OSA and is not FDA-approved in the United States.
To explore its potential benefit, researchers conducted the largest clinical trial to date evaluating sultiame for OSA. The trial included 298 adults across 28 hospitals and community centres in five European countries. Participants, aged 18–75 years with moderate to severe untreated OSA, were randomly assigned to receive placebo or sultiame in doses of 100 mg, 200 mg, or 300 mg. All participants took three visually identical tablets nightly within an hour of bedtime. Sleep assessments, including polysomnography, were carried out at baseline, week 4, and week 15.
The study’s primary endpoint focused on the relative change in the Apnea–Hypopnea Index (AHI3a)—a measure of breathing interruptions during sleep—between baseline and week 15. Secondary measures included absolute change in AHI, oxygen saturation levels, sleep fragmentation, and daytime sleepiness assessed through the Epworth Sleepiness Scale (ESS).
The key findings from the trial were as follows:
- Sultiame demonstrated a clear dose-dependent improvement in OSA outcomes.
- Compared with placebo, AHI3a decreased by 16.4% with the 100 mg dose, 30.2% with the 200 mg dose, and 34.6% with the 300 mg dose.
- Sleep fragmentation was reduced by 5.7 events per hour with the 200 mg dose and 6.7 events per hour with the 300 mg dose.
- Patients receiving 200 mg reported significant improvements in daytime sleepiness.
- No notable differences were observed across groups in quality-of-life scores or heart rate.
- Adverse events increased with rising doses: 73% in the 100 mg group, 84% in the 200 mg group, and 91% in the 300 mg group, versus 61% in the placebo group.
- Frequently reported side effects included paraesthesia, headache, nasopharyngitis, and COVID-19.
- Based on the balance between benefits and side effects, the 200 mg dose was considered the most favourable.
According to the study authors, the findings offer strong justification for advancing sultiame to further clinical development. With consistent improvements observed across breathing indices, nocturnal oxygenation, and sleep quality, the study provides a promising foundation for the first potential pharmacological treatment of OSA.
Reference:
Randerath W, Grote L, Stenlöf K, Fietze I, Chevts J, Buntinx E, Albares J, Kuhn K, Hansen C, Völp A, Hedner J; FLOW study investigators. Sultiame once per day in obstructive sleep apnoea (FLOW): a multicentre, randomised, double-blind, placebo-controlled, dose-finding, phase 2 trial. Lancet. 2025 Oct 25;406(10514):1983-1992. doi: 10.1016/S0140-6736(25)01196-1. Epub 2025 Oct 9. PMID: 41077049.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751
Next Story


