- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
MRI safety software tool receives FDA's qualification for MDDT
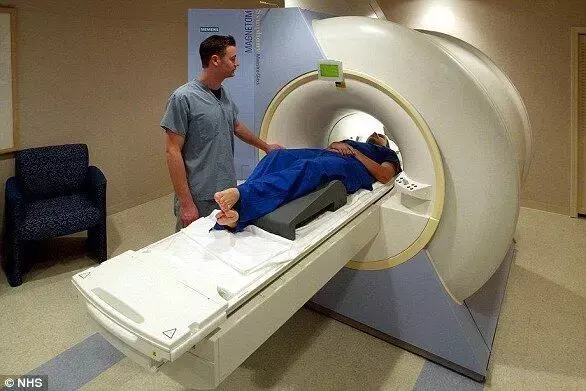
USA: The US Food and Drug Administration (FDA) has given the regulatory go-ahead to a software tool designed to help users analyze the safety of MRI environments.
According to FDA, the tool qualified the Virtual MRI Safety Evaluations of Medical Devices tool under its Medical Device Development Tools (MDDT) program. The tool can help users maintain safety in the MRI environment through the following:
· Predicting interactions between medical device implants and electromagnetic fields in the MRI environment
· Predicting temperature rise in tissue caused by radiofrequency (RF) heating near electrically passive fully implanted medical devices such as orthopedic joint prostheses and cardiovascular stents.
· Generating results more efficiently compared with bench testing medical devices.
Regulatory paperwork for the tool was submitted by MED Institute, which notes that the tool is a nonclinical assessment model (NAM) that uses computer modeling and simulation to predict the interactions of medical devices in the MRI environment.
The MDDT program supports innovation in medical device development and regulatory science, helping to bridge the gap between research of medical devices and delivery of devices to patients. The program provides FDA with qualified tools that medical device sponsors can utilize during the evaluation and development of medical devices. To earn qualification, FDA evaluates the submitted tool and reviews available supporting evidence to determine if the tool can provide scientifically plausible measurements.
In submitting the application to the FDA, MED Institute noted that there has been growth both in the number of MRI scanners in use and the number of patients with implanted medical devices who may require MRI scans at some point during their lives. The tool can help predict whether MRI can be used safely in these patients.
The MDDT program is designed to facilitate the development and regulatory evaluation of medical devices. More information on the MDDT program is available on the FDA's website.
"We would like to thank the FDA for the opportunity to participate in the MDDT program", says David Gross, Director of MRI Safety Evaluations and Engineering Simulations at MED Institute. "We are excited to offer this MDDT to our clients and help them get their products to market faster, at a lower cost, and with better data."
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


