- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Reduced-dose gadobutrol should be considered for contrast-enhanced brain MRI: Study
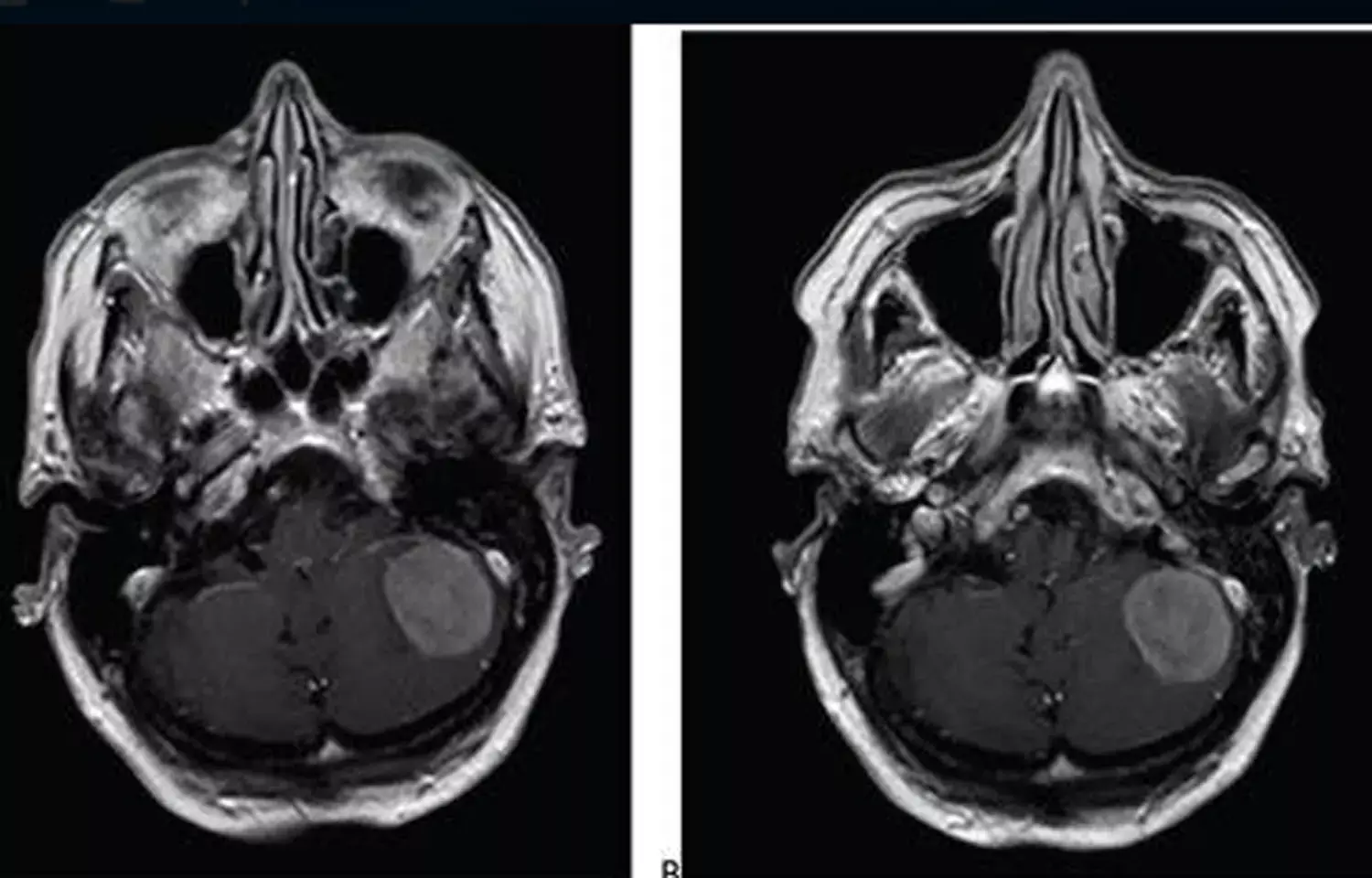
USA: The use of a 25%-reduced dose of gadobutrol (rd-gadobutrol) should be considered for brain MRI, especially in patients undergoing multiple contrast-enhanced examinations, suggests a recent study.
The study, published in the American Journal of Roentgenology (AJR), found rd-gadobutrol to be non-inferior to a 100%-standard dose of gadoterate (sd-gadoterate) for contrast-enhanced brain MRI.
"A 25% reduced gadobutrol dose demonstrated non-inferior efficacy versus standard-dose gadoterate for contrast-enhanced brain MRI," corresponding author Jan Endrikat of Germany's University Medical School of Saarland elaborated, "warranting particular consideration in patients undergoing multiple contrast-enhanced examinations."
In this international, prospective, multicenter, open-label, crossover trial (LEADER-75), 141 patients (78 men, 63 women; mean age, 58.5 years) with known or suspected CNS pathology underwent contrast-enhanced brain MRI with standard-dose gadoterate (0.1 mmol/ kg). A second MRI with reduced-dose gadobutrol (0.075 mmol/kg) was performed within 15 days if an enhancing lesion was identified.
Key findings of the study include:
- Improvement of rd-gadobutrol over unenhanced images was non-inferior to improvement of sd-gadoterate over unenhanced images using 20% non-inferiority margin for all three primary efficacy measures using mean readings.
- In post-hoc analysis, mean reading for the three primary efficacy measures differed by less than 1% between rd-gadobutrol and sd-gadoterate, supporting equivalence of all measures using a narrow ±5% margin.
- Total lesions detected by mean reading was 301 for rd-gadobutrol versus 291 for sd-gadoterate.
- Mean confidence was 3.3±0.6 for rd-gadobutrol versus 3.3±0.6 for sd-gadoterate.
- Sensitivity (58.7%), specificity (91.8%), and accuracy (70.2%) for malignancy from majority reading were identical for rd-gadobutrol and sd-gadoterate.
- Reader preference was not different.
Comparison of reduced-dose gadobutrol and standard-dose gadoterate versus unenhanced imaging demonstrated noninferiority using a 20% margin for three primary efficacy measures: subjective lesion enhancement, lesion border delineation, lesion internal morphology.
"Various secondary variables also supported non-inferiority of reduced-dose gadobutrol," the authors of the AJR article added.
Reference:
The study titled, "Clinical Efficacy of Reduced Dose Gadobutrol Versus Standard Dose Gadoterate for Contrast-Enhanced MRI of the CNS: An International Multicenter Prospective Crossover Trial (LEADER-75)," is published in the American Journal of Roentgenology.
DOI: https://www.ajronline.org/doi/10.2214/AJR.21.25924
Hina Zahid Joined Medical Dialogue in 2017 with a passion to work as a Reporter. She coordinates with various national and international journals and association and covers all the stories related to Medical guidelines, Medical Journals, rare medical surgeries as well as all the updates in the medical field. Email: editorial@medicaldialogues.in. Contact no. 011-43720751
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


