- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Strides Shasun gets DCGI nod to manufacture Hepatitis C drug
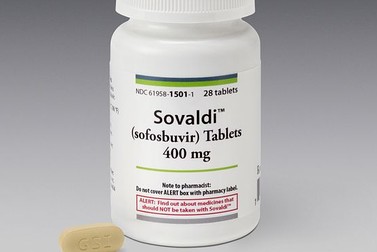
New Delhi: Drug firm Strides Shasun has received approval from the Drug Controller General of India (DCGI) to produce a generic version of Gilead's 'Sovaldi' used for treatment of Hepatitis C.
The company has received approval for manufacturing a generic version of Sofosbuvir 400 mg, Strides Shasun said in a filing to BSE.
Last year, Strides had entered into a licensing agreement with Gilead Sciences to bring Hepatitis C cure to 91 developing countries.
"The product will continue to be marketed under the brand name Virso in India and overseas," Strides Shasun said.
Sofosbuvir is the first-in-class polymerase inhibitor to be launched in India for Hepatitis C treatment and represents "a paradigm shift" in the existing Hepatitis C cure, it added.
"The high potency, high barrier to resistance and pan genotype activity make Sofosbuvir a breakthrough drug in Hepatitis C treatment. This drug in combination therapy has shown to have high cure rates of around 90 per cent," it said.
Globally, it is estimated that 170-185 million people are chronically infected with Hepatitis C virus. In India alone, it is estimated that 12-18 million patients are infected with the disease, which is several fold higher than those with HIV/AIDS.
Next Story


