- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
SCAI releases 2023-Expert consensus statement on transcatheter LAA closure
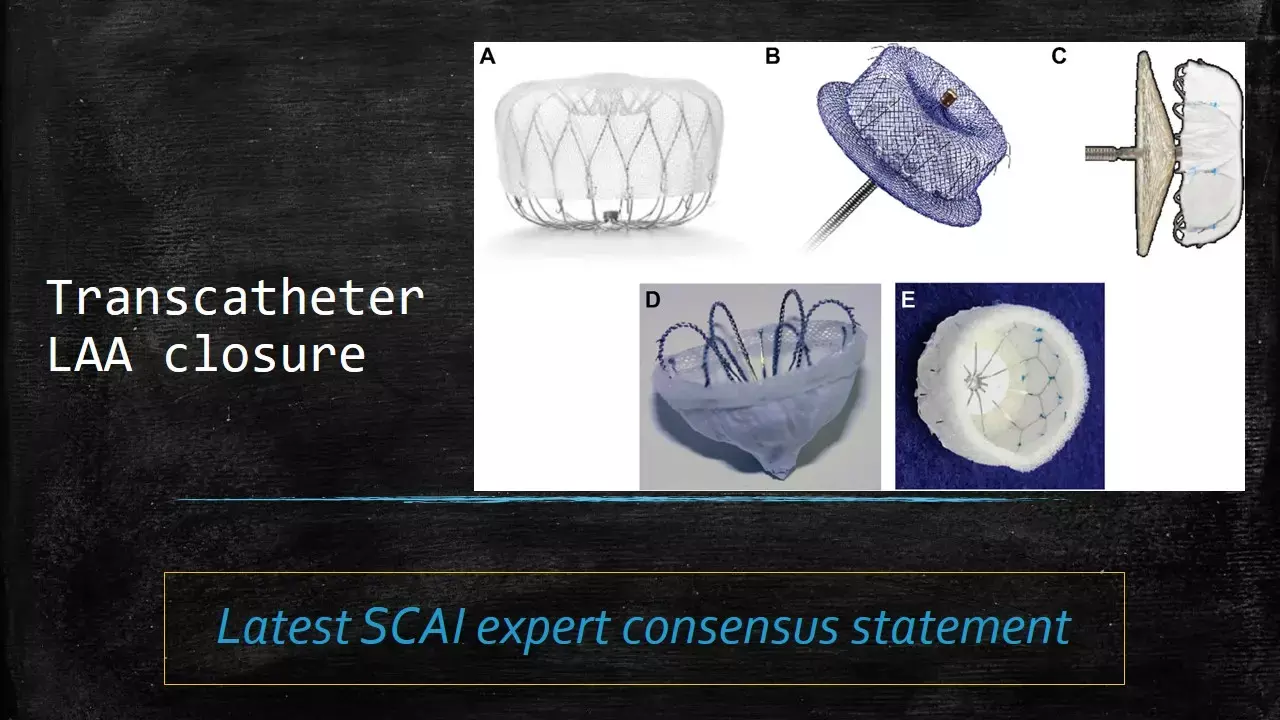
Over the past 2 decades, the field of transcatheter endovascular left atrial appendage closure (LAAC) has rapidly expanded, with a myriad of devices approved or in clinical development. After FDA approval of WATCHMAN device in 2015, the number of LAAC procedures have risen exponentially.
Since the release of SCAI consensus statements in 2015 and 2016, many new trials and registries have been published and thus an updated consensus statement was released recently by SCAI to provide recommendations on contemporary, evidence-based best practices for transcatheter LAAC focusing on endovascular devices. These recommendations were recently published in JACC CI.
Following is the summary of these recommendations:
1. Indications of LAAC: Transcatheter LAAC is appropriate for patients with nonvalvular atrial fibrillation with high thromboembolic risk who are not suited for long-term oral anticoagulation and who have adequate life expectancy (minimum >1 year) and quality of life to benefit from LAAC.
2.1. Training: Physicians performing LAAC should have a prior experience, including ≥50 prior left-sided ablations or structural procedures and ≥25 transseptal punctures (TSPs).
Interventional imaging physicians should have experience in guiding ≥25 TSPs before supporting any LAAC procedures independently.
2.2. For maintenance of skills, implanting physicians should perform ≥25 TSPs and >12 LAACs over each 2-year period.
2.3. New programs and implanting physicians early in their LAAC experience should have on-site cardiovascular surgery backup.
3. Baseline imaging with TEE or cardiac CT is recommended before LAAC.
4. Intraprocedural imaging guidance with TEE or intracardiac echocardiography is recommended.
5. Technical aspects of the procedure, including venous access, anticoagulation, transseptal puncture, delivery sheath selection and placement, left atrial pressure measurement, and device deployment, should be performed in accordance with the labeling of each specific LAAC device.
6. Operators need to be familiar with avoidance, recognition, and management of procedural complications associated with LAAC.
7. Predischarge imaging should be performed with 2-dimensional TTE to rule out pericardial effusion and device embolization. Same-day discharge may be appropriate after several hours of observation demonstrating no complications or pericardial effusion after LAAC.
8. Device-related thrombus should be treated with anticoagulation. Repeat imaging at 45- to 90-day intervals can be performed to assess for resolution with eventual cessation of anticoagulation.
9. Routine closure of iatrogenic atrial septal defects associated with LAAC should not be performed.
10. The clinical impact and management of peridevice leaks are not fully understood, and all efforts should be made to minimize such leaks at the time of implantation.
11. Patients should be prescribed antithrombotic therapy with warfarin, direct oral anticoagulants, or dual antiplatelet therapy after LAAC according to the studied regimen and instructions for use for each specific device and tailored to the bleeding risks of each patient.
12. TEE or cardiac CT is recommended at 45 to 90 days after LAAC for device surveillance to assess for peridevice leak and device-related thrombus.
13. Combined procedures with LAAC (eg, structural interventions, pulmonary vein isolation) are not routinely recommended, as data are pending from ongoing randomized controlled trials.
The FDA approval of WATCHMAN FLX and Amulet devices, in-progress RCTs evaluating new devices, and an array of other technologies in the development and approval queue will mean a larger toolbox for LAAC.
Several RCTs comparing LAAC to controls in OAC-ineligible patients and RCTs comparing DOAC to LAAC in OAC-eligible patients are ongoing and are anticipated to broaden the clinical indications and strengthen societal recommendations for this device therapy.
This document provides the current evidence-based best practices of endovascular LAAC by consensus of the established expert panel with multisocietal support.
Source: JACC CI: DOI: 10.1016/j.jcin.2023.01.011
MBBS, MD , DM Cardiology
Dr Abhimanyu Uppal completed his M. B. B. S and M. D. in internal medicine from the SMS Medical College in Jaipur. He got selected for D. M. Cardiology course in the prestigious G. B. Pant Institute, New Delhi in 2017. After completing his D. M. Degree he continues to work as Post DM senior resident in G. B. pant hospital. He is actively involved in various research activities of the department and has assisted and performed a multitude of cardiac procedures under the guidance of esteemed faculty of this Institute. He can be contacted at editorial@medicaldialogues.in.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


