- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Catheter-based renal artery denervation shows long-term positive outcomes for resistant hypertension: SYMPLICITY HTN-3 Trial
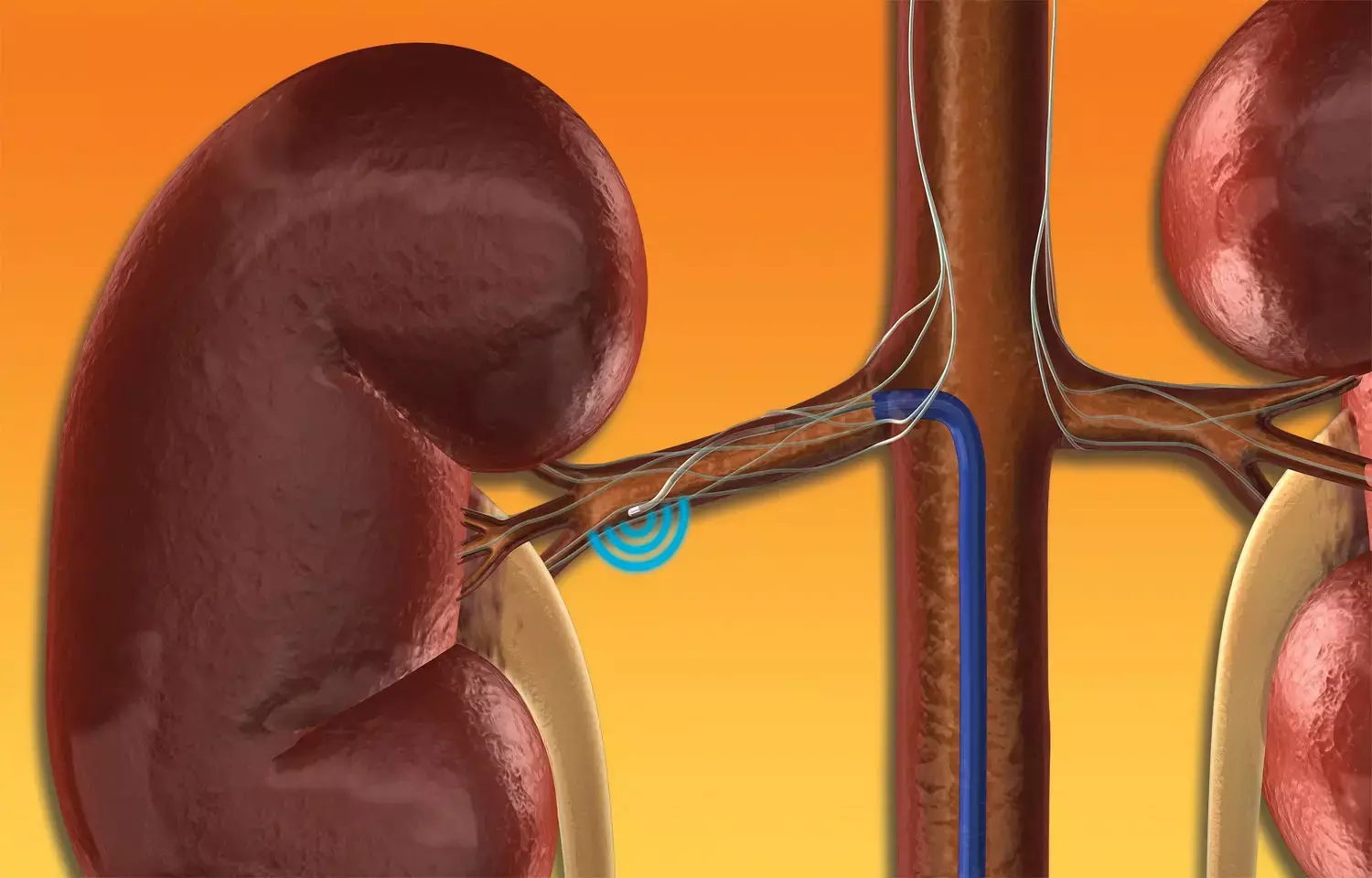
USA: The Renal Denervation in Patients With Uncontrolled Hypertension -- SYMPLICITY HTN-3 trial demonstrated the safety of the Symplicity system but not efficacy at six months of follow-up in treatment-resistant hypertension. The final report of the trial, published in The Lancet on September 18, 2022, presents the results of a 36-month follow-up.
The study showed sustained reductions in diastolic and systolic blood pressure (BP) in patients treated with the Symplicity Flex renal denervation catheter (Medtronic) versus those treated with a sham procedure. It adds to the totality of evidence supporting the safety of renal artery denervation 36 months following the process.
"Patients who were randomly assigned originally to receive renal artery denervation experienced a larger reduction in BP and better BP control versus the patients who received sham control from 12 months to 36 months," Prof Deepak L Bhatt, Brigham and Women's Hospital Heart and Vascular Center and Harvard Medical School, Boston, MA, USA, and colleagues wrote in their study.
SYMPLICITY HTN-3 is a multicentre, single-blind, sham-controlled, randomized controlled trial performed in 88 centers in the USA. The trial included adults aged 18–80 with treatment-resistant hypertension on stable, maximally tolerated doses of three or more drugs, including a diuretic with a seated office systolic BP of 160 mm Hg or more and 24 h ambulatory systolic BP of 135 mm Hg or more. The patients were randomly assigned in a 2:1 ratio to receive renal artery denervation through the single electrode (Flex) catheter or sham control.
The original endpoint was the office systolic blood pressure change from baseline to 6 months for the renal artery denervation group versus the sham control group. After the primary endpoint assessment at six months, patients were unmasked. At that point, eligible patients in the sham control group who met the inclusion criteria could cross over to receive renal artery denervation.
The researchers analyzed changes in blood pressure up to 36 months in patients placed in the original renal artery denervation group versus the sham control group; this included the crossover group, those who underwent renal artery denervation after six months, and the non-crossover group, those who did not.
For comparing the renal artery denervation and sham control groups, the researchers attributed values of follow-up blood pressures for patients in the crossover group utilizing the most current pre-crossover masked blood pressure value. The primary safety endpoint was an end-stage renal disease, all-cause mortality, vascular complications, renal artery perforation or dissection requiring intervention, significant embolic event, new renal artery stenosis of more than 70%, or hospitalization for hypertensive crisis.
The researchers screened 1442 patients from September 29, September 29, 2011, to May 6, May 6, 2013, of whom 535 were randomly assigned: 364 patients received renal artery denervation, and 171 received the sham control. The follow-up data for 36 months was available for original renal artery denervation group (n=219), crossover group (n=63 patients), and non-crossover group (n=33 patients).
The study led to the following findings:
· At 36 months, the change of office systolic BP was –26·4 mm Hg in the renal artery denervation group, and the sham control group, it was –5·7 mm Hg.
· At 36 months, the change in 24 h ambulatory systolic blood pressure was –15·6 mm Hg in the renal artery denervation group and –0·3 mm Hg in the sham control group.
· The renal artery denervation group without imputation spent a considerably longer time in the therapeutic blood pressure range (i.e., better blood pressure control) than patients in the sham control group [renal artery denervation group (18%) vs. sham control group (9%)} despite a comparable medication burden, with significant and consistent results with imputation.
· Adverse events rates were comparable across treatment groups, with no evidence of late-emerging complications from renal artery denervation.
· At 48 months, the rate of the composite safety endpoint to 48 months, including all-cause death, new-onset end-stage renal disease, a significant embolic event resulting in end-organ damage, vascular complication, renal artery re-intervention, and hypertensive emergency was 15% for the renal artery denervation group, 14% for the crossover group, and 14% for the non-crossover group.
"Our findings from the long-term follow-up of the SYMPLICITY HTN-3 trial adds to the evidence supporting the safety of renal artery denervation to 36 months following the procedure," the researchers wrote.
"From 12 months to 36 months following the procedure, patients in the renal artery denervation group had larger decreases in blood pressure and better blood pressure control versus patients who received sham control," they conclude.
Reference:
The study titled "Long-term outcomes after catheter-based renal artery denervation for resistant hypertension: final follow-up of the randomized SYMPLICITY HTN-3 Trial" was published in The Lancet.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


