- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Concomitant LAAO an alternative to medical therapy for AF patients undergoing TAVR: WATCH-TAVR trial
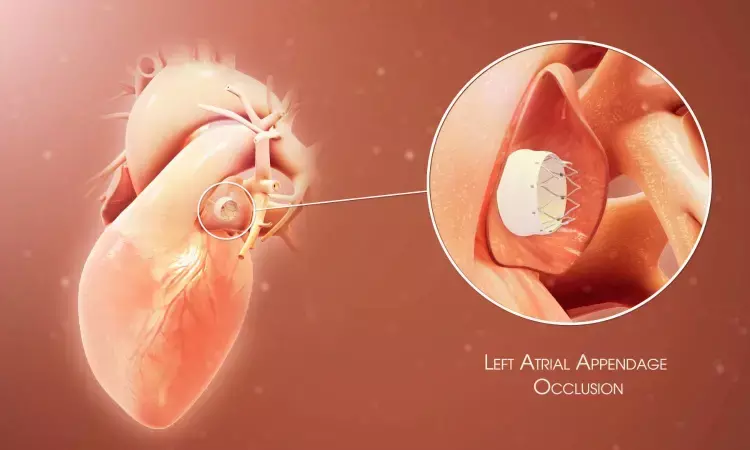
USA: Concurrent left atrial appendage occlusion (LAAO) and transcatheter aortic valve replacement (TAVR) is a noninferior treatment option to TVR plus medical therapy in severe aortic stenosis patients with atrial fibrillation, a recent study has shown.
The researchers advise considering the increased complexity and risks of the combined procedure when concomitant LAAO is viewed as an alternative for medical therapy for patients with AF undergoing TAVR. The findings from the WATCH-TAVR study were presented at TCT 2023 and simultaneously published in Circulation.
"Concomitant LAAO with the Watchman 2.5 device (Boston Scientific) and TAVR was noninferior to TAVR plus chronic oral anticoagulation for the primary endpoint of stroke, all-cause mortality, and major bleeding at 2 years among patients with severe symptomatic aortic stenosis and AF," the researchers reported.
At 2-year follow-up, the primary noninferiority endpoint occurred in 33.9% of patients who received LAAO a TAVR compared with 37.2% who received TAVR plus medical therapy (HR = 0.86;).
In patients undergoing TAVR, atrial fibrillation is common and is associated with an increased risk of stroke and bleeding. Left atrial appendage occlusion is being approved as an alternative to anticoagulants for stroke prevention in AF patients but placement of these devices neither in patients with severe AS nor at the same time as TAVR, has been extensively studied.
To fill this knowledge gap, Samir R. Kapadia, Cleveland Clinic, Cleveland, OH, and colleagues evaluated the effectiveness and safety of concomitant TAVR and LAAO with WATCHMAN in AF patients by conducting WATCH-TAVR, a multicenter, randomized trial.
349 patients were randomized in the ratio of 1:1 to TAVR+LAAO (n=177) or TAVR+medical therapy (n=172). WATCHMAN patients received anticoagulation for 45 days followed by dual antiplatelet therapy (DAPT) until 6 months. For patients randomized to medical therapy, anticoagulation was per the treating physician's preference. The primary non-inferiority endpoint was stroke, all-cause mortality, and major bleeding at 2 years between the two strategies.
The researchers reported the following findings:
· CHA2DS2-VASc score was 4.9 and HAS-BLED score was 3.0.
· At baseline, 85.4% of patients were taking anticoagulation and 71.3% of patients were on antiplatelet therapy. The cohorts were well-balanced for baseline characteristics.
· The incremental LAAO procedure time was 38 minutes; the median contrast volume was 119 mL for combined procedures versus 70 mL with TAVR alone.
· At 24 months of follow-up, 82.5% compared to 50.8% of patients were on any antiplatelet therapy, and 13.9% compared to 66.7% of patients were on any anticoagulation therapy in TAVR+LAAO compared to TAVR+medical therapy group respectively.
· For the composite primary endpoint, TAVR+.LAAO was non-inferior to TAVR+ medical therapy (22.7 vs 27.3 events/100 patient years for TAVR+LAAO and TAVR+medical therapy respectively; Hazard Ratio 0.86).
"The risks of the combined procedures and increased complexity should be considered when concomitant LAAO is viewed as an alternative to medical therapy for patients with AF undergoing TAVR," the researchers wrote.
The study did not account for differences between the groups beyond 2 years. Also, the study was limited to using only the Watchman 2.5 device.
Reference:
Kapadia SR, et al. Circulation. 2023;doi:10.1161/CIRCULATIONAHA.123.067312.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


