- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Coronary sinus reducer improves symptoms in patients with refractory angina: ORBITA-COSMIC trial
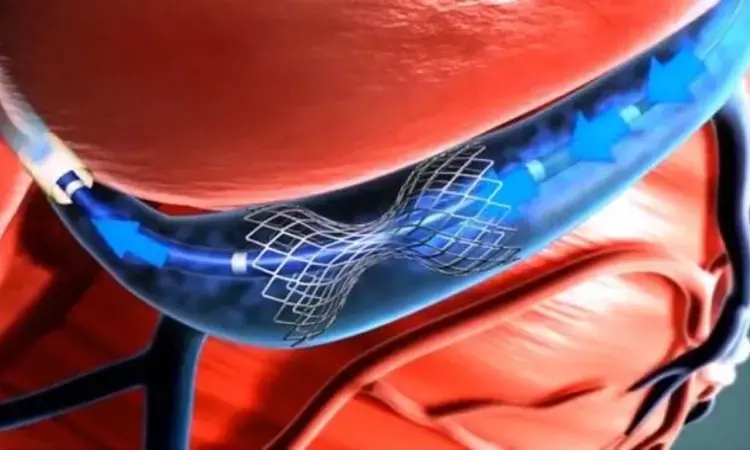
UK: A coronary sinus reducer (CSR) implant showed a symptom alleviation benefit for people with stable coronary artery disease and refractory angina, according to a small placebo-controlled ORBITA-COSMIC trial.
"The number of self-reported daily angina episodes shifted downward significantly among people randomized to the Neovasc Reducer intervention instead of a sham procedure (OR 1.40)," the researchers reported at the 2024 American College of Cardiology (ACC) conference. The findings were published in The Lancet.
However, despite the purported mechanism of benefit of the device, there was no improvement in myocardial blood flow in ischemic segments with the coronary sinus reducer over placebo (difference 0.06 ml/min/g).
The coronary sinus reducer is an hourglass-shaped stainless-steel mesh percutaneously implanted in the coronary sinus to reduce angina. It is the only antianginal therapy that acts on the cardiac venous circulation and is proposed to reduce angina in patients with stable coronary artery disease by improving myocardial perfusion.
Michael J Foley, Imperial College Healthcare NHS Trust, London, UK, and colleagues aimed to measure the efficacy of CSR implant, compared with placebo, on myocardial ischemia reduction and symptom improvement.
For this purpose, the researchers conducted the ORBITA-COSMIC trial at six UK hospitals. Patients aged 18 years or older with angina, ischemia, stable coronary artery disease, and no further options for treatment were eligible. Before entering a 2-week symptom assessment phase, in which patients reported their angina symptoms using a smartphone application (ORBITA-app), all patients completed a quantitative adenosine-stress perfusion cardiac magnetic resonance (CMR) scan, symptom and quality-of-life questionnaires, and a treadmill exercise test.
Patients were randomly assigned in a 1:1 ratio to receive either CSR or placebo. Following the CSR implantation or placebo procedure, patients entered a 6-month blinded follow-up phase in which they reported their daily symptoms in the ORBITA app. All assessments were repeated at six months.
The study's primary outcome was myocardial blood flow in segments designated ischaemic at enrolment during the adenosine-stress perfusion CMR scan. The primary symptom outcome was the number of daily angina episodes.
Following were the study’s key findings:
· Between May 26, 2021, and June 28, 2023, 61 patients were enrolled between 2021 and 2023, of whom 51 (86%] male) were randomly assigned to either the CSR group (n=25) or the placebo group (n=26). Of these, the intention-to-treat analysis included 50 patients (24 in the CSR group, and 26 in the placebo group).
· 57% of 800 imaged cardiac segments were ischaemic at enrolment, with a median stress myocardial blood flow of 1·08 mL/min per g.
· Myocardial blood flow in ischaemic segments did not improve with CSR compared with placebo.
· The number of daily angina episodes was reduced with CSR compared with placebo (OR 1·40).
· There were two CSR embolization events in the CSR group, and no acute coronary syndrome events or deaths in either group.
ORBITA-COSMIC trial did not confirm the prespecified hypothesis that CSR's mechanism of action consists of an increase of perfusion in ischaemic myocardial segments. However, the imaging data suggested a possible redistribution of perfusion towards the subendocardium in ischaemic segments.
The CSR, however, produced a clear reduction in angina frequency reported by patients, which developed gradually over weeks.
"These data provide evidence for CSR use as an antianginal therapeutic option for patients with refractory angina, myocardial ischemia, and stable coronary artery disease," the researchers concluded.
Reference:
Foley MJ, et al "Coronary sinus reducer for the treatment of refractory angina: a randomised, placebo-controlled trial (ORBITA-COSMIC)" ACC 2024.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


