- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
FDA OKs first renal denervation device for hypertension treatment
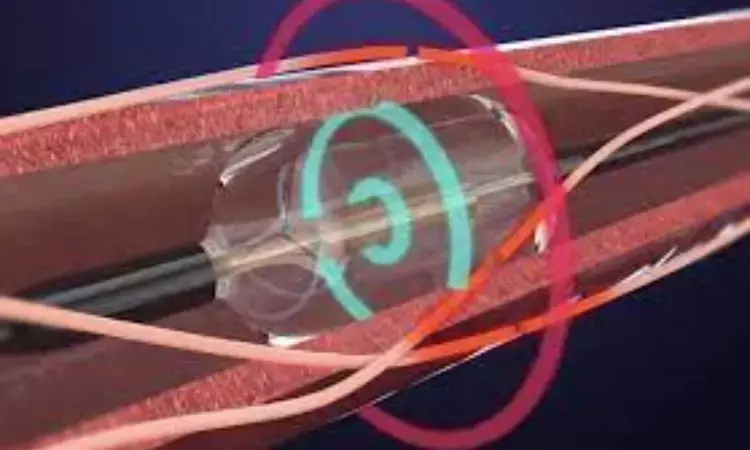
USA: The US Food and Drug Administration (FDA) has approved Recor's Paradise Ultrasound Renal Denervation (RDN) system for hypertension treatment. The approval makes innovative hypertension treatment available for the first time in the US.
The Paradise device, which delivers ultrasound pulses through each of the main renal arteries to the surrounding nerves, is indicated when lifestyle changes and medications have not adequately controlled hypertension.
Approval of the Paradise system follows Recor’s positive FDA Advisory Committee Panel in August 2023. Earlier this year, results from Recor’s US pivotal study, the RADIANCE™ II Randomized Clinical Trial, were published in the Journal of the American Medical Association (JAMA). The Paradise Ultrasound RDN system met the primary safety and effectiveness endpoints without major adverse events.
The Paradise system is intended as an adjunctive treatment option when medications and lifestyle changes have not adequately controlled a patient’s blood pressure (BP). It is a first-of-its-kind ultrasound-based RDN technology designed for BP lowering by denervating the sympathetic nerves surrounding the renal arteries, reducing the overactivity that can lead to hypertension.
The Paradise system delivers two to three doses of 360-degree ultrasound energy — lasting seven seconds each — through each of the main renal arteries to the surrounding nerves. The Paradise catheter features the exclusive HydroCooling™ system, which circulates sterile water through the balloon catheter during the procedure to help protect the renal artery wall.
“Recor is leading the way in bringing an innovative solution to clinicians and their patients struggling to control blood pressure. This FDA approval is the culmination of years of technical research and rigorous clinical studies,” Lara Barghout, President and CEO of Recor Medical said in a press release. “We are grateful to the patients who participated in the studies and to the clinical trial investigator teams whose diligence and dedication made FDA approval possible. We look forward to making this technology available to physicians and their patients nationwide.”
Hypertension is the leading contributor to disease burden worldwide, resulting in increased cardiovascular morbidity and mortality, poor quality of life, and higher costs to health systems. The Paradise Ultrasound RDN system previously received a CE mark has been successfully introduced in Europe and is an investigational device in Japan.
“Despite the longstanding availability of dozens of affordable anti-hypertensive medications, blood pressure control rates in the United States are alarmingly low and falling. Given the significant blood pressure reductions seen in the ultrasound renal denervation trials, the Paradise Ultrasound Renal Denervation system offers a much-needed advancement in our currently available options to control hypertension,” said site principal investigator Naomi Fisher, MD, Associate Professor of Medicine, Harvard Medical School, and Director of Hypertension Service and Hypertension Innovation, Division of Endocrinology, Diabetes and Hypertension at Brigham and Women’s Hospital.
“uRDN has proven efficacy in patients with truly resistant hypertension, a population for whom medication therapy often fails. It is also effective in patients with mild to moderate hypertension who cannot tolerate enough medication to control their blood pressure.”
“Approval of the Paradise Ultrasound RDN system marks an important milestone for the company and provides a new adjunctive treatment option for hypertension which remains inadequately controlled despite conventional therapies,” said Noriko Tojo, President and Representative Director of Otsuka Medical Devices and Executive Director of Otsuka Holdings Co., Ltd.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


