- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
"Leadless" leading the way ahead - JAMA study provides reassuring results for leadless pacemakers.
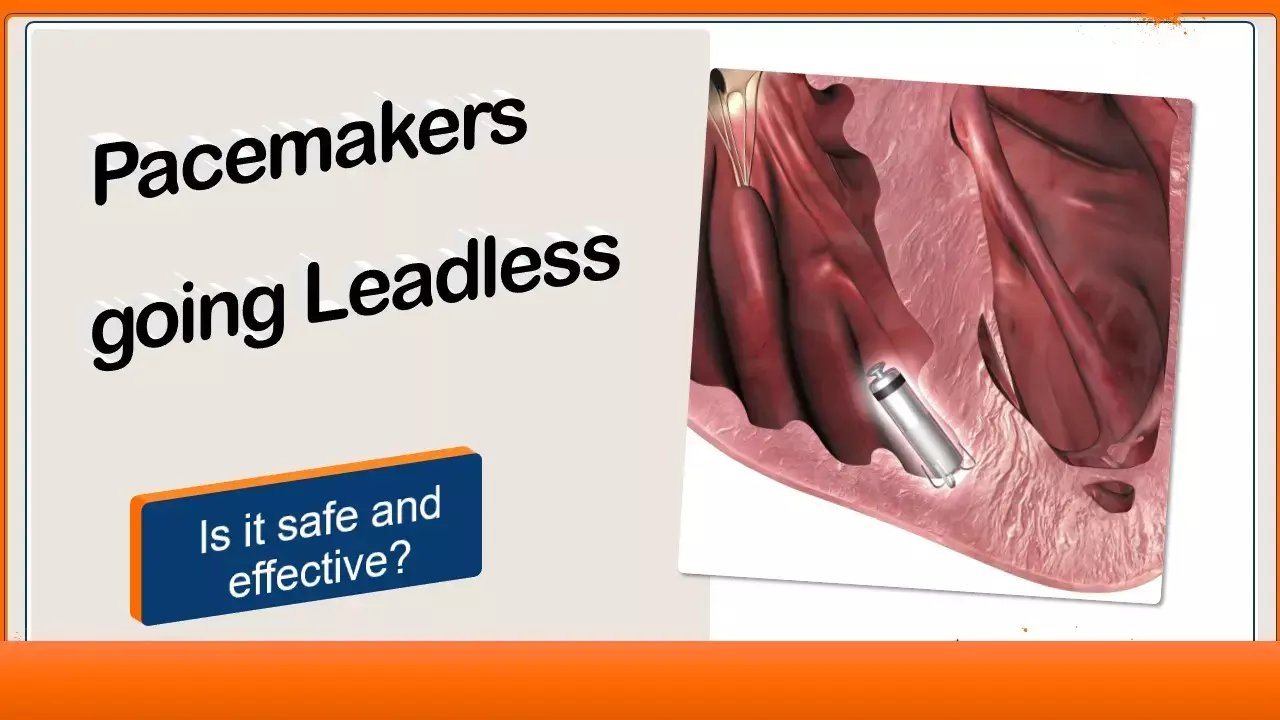
The safety and efficacy of leadless VVI pacemakers have been demonstrated in multiple clinical trials, but the comparative performance of the device in a large, real-world population has not been examined. Piccini et al in a pivotal cohort study published in JAMA cardiology have now shown that patients implanted with leadless pacemakers have lower rates of other device-related complications and requirements for device revision at 6 months compared to transvenous implants.
Understanding the benefits and risks associated with leadless VVI pacemakers compared with transvenous VVI pacemakers can help clinicians and patients make informed treatment decisions.
Leadless technology was designed to reduce complications related to transvenous pacing leads and subcutaneous pockets—the most common sources of transvenous pacemaker-related complications.
The objective of this analysis is to compare the safety and complication rates of patients treated with leadless VVI pacemakers with a contemporary cohort of patients treated with transvenous VVI pacemakers.
The Micra CED is a continuously enrolling observational cohort study evaluating complications, utilization, and outcomes of leadless VVI pacemakers. The main outcomes were acute (30-day) complications and 6-month complications. This comparison has generated following findings:
1. Despite significant differences in patient characteristics, the rate of the complications measured through 6 months was significantly lower in patients who were implanted with leadless VVI pacemakers after adjustment.
2. Patients with a leadless VVI pacemaker had a substantially lower rate of device revision in the 6 months after implantation.
3. Finally, although the adjusted risk of cardiac effusion and/or perforation at 30 days was higher with leadless VVI pacing, device-related complications were significantly lower.
4. It is important to note that the adjusted rate of pericardial effusion and/or perforation was higher with leadless VVI pacemaker implantation compared with transvenous VVI pacemaker implantation (0.8% vs 0.4%); however the absolute rate was less than 1%.
This report provides an initial assessment of outcomes in this ongoing study and a direct comparison between patients with leadless VVI pacemakers and patients with transvenous VVI pacemakers in the Medicare setting.
Despite the older patient age and higher comorbidity burden, outcomes of leadless pacing observed in the investigational device exemption and postapproval registry trials appear to be maintained in this study of general clinical practice.
The results from this study further develop the evidence on leadless pacemakers in practice and can inform shared decision-making about device choice for patients and physicians. Additional studies are needed to ascertain the long-term impact of these differences in net clinical benefit in VVI pacemaker populations.
Source: JAMA Cardiology: doi:10.1001/jamacardio.2021.2621
MBBS, MD , DM Cardiology
Dr Abhimanyu Uppal completed his M. B. B. S and M. D. in internal medicine from the SMS Medical College in Jaipur. He got selected for D. M. Cardiology course in the prestigious G. B. Pant Institute, New Delhi in 2017. After completing his D. M. Degree he continues to work as Post DM senior resident in G. B. pant hospital. He is actively involved in various research activities of the department and has assisted and performed a multitude of cardiac procedures under the guidance of esteemed faculty of this Institute. He can be contacted at editorial@medicaldialogues.in.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


