- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
ESMO 2025 Update: Effect of Abemaciclib added to standard Endocrine Therapy in High-Risk Early Breast Cancer

Adjuvant abemaciclib combined with endocrine therapy (ET) has demonstrated a statistically significant and clinically meaningful improvement in overall survival (OS) compared with standard ET in patients with hormone receptor–positive (HR+), human epidermal growth factor receptor 2–negative (HER2−), node-positive, high-risk early breast cancer (EBC), as reported in the recently...
Adjuvant abemaciclib combined with endocrine therapy (ET) has demonstrated a statistically significant and clinically meaningful improvement in overall survival (OS) compared with standard ET in patients with hormone receptor–positive (HR+), human epidermal growth factor receptor 2–negative (HER2−), node-positive, high-risk early breast cancer (EBC), as reported in the recently published Phase 3 monarchE trial.
The study published in the October 2025 issue of Annals of Oncology highlighted a sustained invasive disease-free survival (IDFS) and distant relapse-free survival (DRFS) benefit with adjuvant abemaciclib-ET at 7 years.
Abemaciclib, a cyclin-dependent kinase (CDK) 4/6 inhibitor(i), is approved for high-risk EBC and advanced breast cancer in both first and second-line settings. Two years of adjuvant abemaciclib-ET is the standard of care for patients with HR+, HER2−, node-positive, high-risk EBC. In the monarchE trial, at 54 months, abemaciclib-ET showed sustained, clinically meaningful improvements in IDFS and DRFS. Although OS remains the gold standard for assessing both efficacy and safety of cancer therapies, OS events in HR+ breast cancer occur gradually. The present monarchE trial reported the primary OS results with updated IDFS and DRFS at a 6.3-year median follow-up.
monarchE is a global, randomized, open-label, Phase 3 study evaluating the efficacy and safety of abemaciclib-ET versus standard ET in adults with HR+, HER2−, node-positive, high-risk EBC. A total of 5637 patients were randomized 1:1 to receive at least 5 years of ET with abemaciclib (n=2808) or without abemaciclib (n=2829) for a 2-year treatment across two cohorts. The majority of the patients fell into Cohort 1 (n=5120; 91%), comprising those with either ≥4 positive pathological axillary lymph nodes or 1–3 nodes with Grade 3 disease and/or tumor ≥5 cm. Cohort 2 (n=517; 9%) included patients with 1–3 positive pathological axillary lymph nodes, tumor grade < 3, tumor size < 5 cm, and central Ki-67 ≥20%. The intent-to-treat (ITT) population comprised all patients enrolled across both cohorts.
The primary efficacy endpoint was IDFS, defined per the Standardized Definitions for Efficacy End Points (STEEP) system. Key secondary efficacy endpoints included DRFS and OS. Safety endpoints were treatment-emergent adverse events (TEAEs), all serious adverse events (SAEs) up to 5 years post-randomisation, hospitalisations, and clinical laboratory abnormalities.
Cohort 1 findings from the study include:
In the ITT population, the addition of abemaciclib to ET resulted in a 15.8% reduction in the risk of death versus ET (HR 0.842, 95% CI 0.722–0.981; two-sided P = 0.027). In Cohort 1, OS, IDFS, and DRFS outcomes were consistent with those observed in the overall ITT population.
1. Efficacy in Cohort 1: OS, IDFS, and DRFS were consistent across the Cohort 1 population.
- Total deaths that occurred were 630 [286 (11.2%) vs 344 (13.4%)] (abemaciclib-ET vs ET arm), which reflects a 16.5% lower death risk in abemaciclib-ET compared to ET.
- The 7-year IDFS and DRFS rates were 77.0% vs 70.1% and 79.5% vs 74.0% (abemaciclib-ET vs ET; P<0.0001).
- Benefits with abemaciclib-ET were consistent across subgroups and Ki-67 index levels, with most deaths due to recurrence. (Fig 1)
2. Safety: The safety profile remained unchanged from previous analyses.
- From all patients completed the 2-year treatment approximately four years earlier, no new discontinuations or dose adjustments were reported.
- During long-term follow-up (over 30 days post-treatment discontinuation), only non-serious adverse events (AEs) related to study treatment and serious adverse events (SAEs) of any cause were recorded.
- SAE incidence was slightly lower with abemaciclib-ET (7.5%) than with ET alone (8.1%).
3. Post-discontinuation anticancer therapies: After completing study treatment, most patients without distant recurrence continued ET (97.0% vs 96.7%; abemaciclib-ET vs ET), with 85.4% vs 82.4% receiving ≥5 years of ET, respectively.
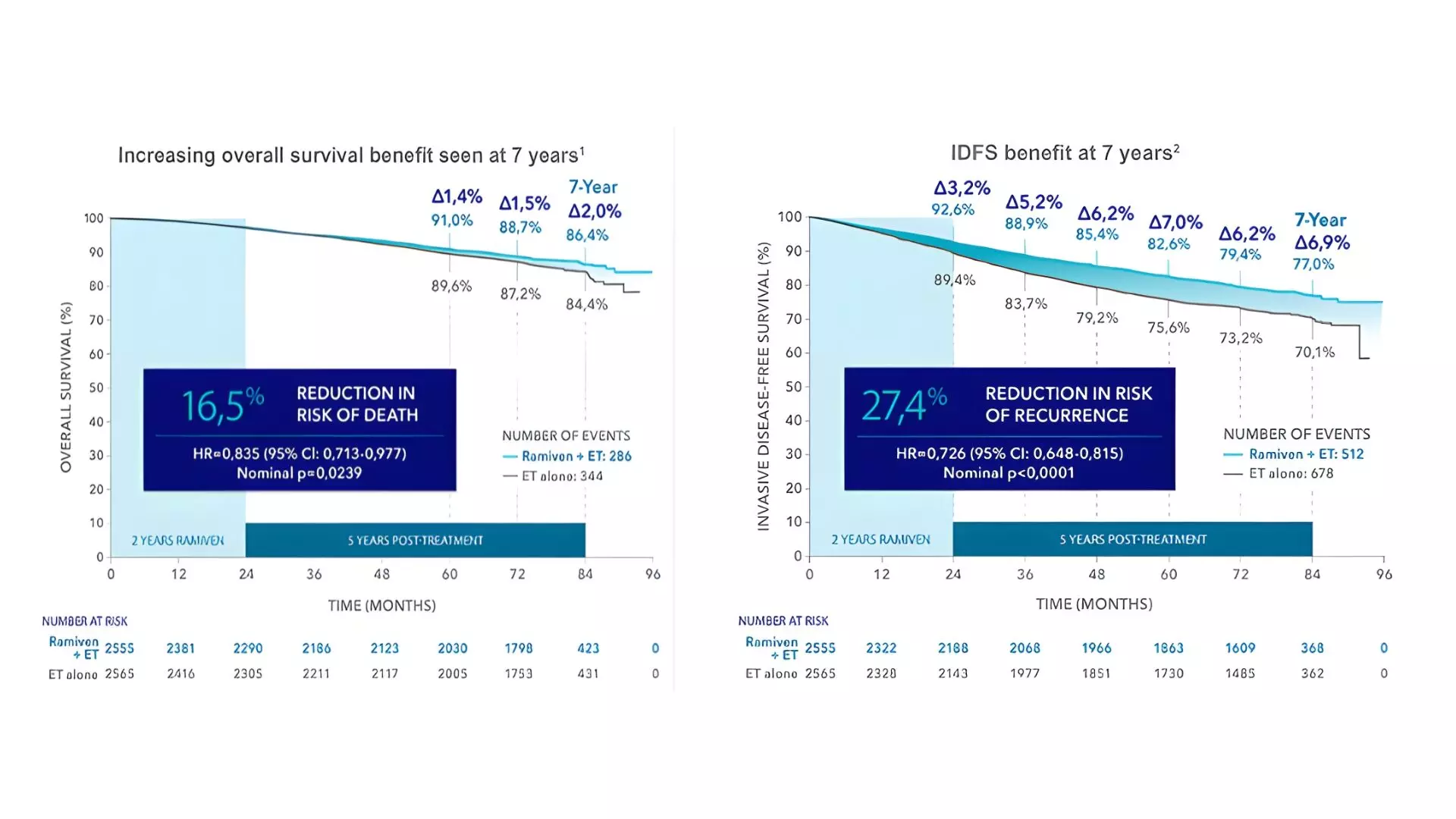
Fig 1. Overall Survival (OS) and Invasive Disease-Free Survival (IDFS) at 7 years (Cohort 1)
In Cohort 2, OS events were similar between abemaciclib-ET and ET (15 [5.9%] vs 16 [6.1%]), while abemaciclib-ET showed numerically favorable IDFS and DRFS trends despite the small sample.
Adding 2 years of abemaciclib to adjuvant ET led to a statistically significant and clinically meaningful improvement in OS compared with ET alone in patients with HR+, HER2−, node-positive, high-risk EBC. At 7 years, abemaciclib-ET maintained durable benefits in IDFS and DRFS. Abemaciclib is the first CDK4/6 inhibitor to show that reducing recurrence risk translates into a significant improvement in OS.
Reference: Johnston S, Martin M, O’Shaughnessy J, Hegg R, Tolaney SM, Guarneri V, Del Mastro L, Campone M, Sohn J, Boyle F, Cortes J, Rugo HS, Goetz MP, Hamilton EP, Huang CS, Senkus E, Cicin I, Testa L, Neven P, Huober J, Shao Z, Wei R, Munoz M, San Antonio B, Shahir A, Rastogi P, Harbeck N, Overall Survival with Abemaciclib in Early Breast Cancer, Annals of Oncology (2025), doi: https://doi.org/10.1016/j.annonc.2025.10.005.
PP-AL-IN-1773 | 12/12/2025 Exp date – 10 Dec 2027
Disclaimer
Eli Lilly and Company (India) Private Limited
For further Information about Lilly and Lilly products please contact us at the below address: Plot 92, Sector 32 Gurgaon, Haryana, 122001, India Ph.: +91-124-4753000/01 | www.lilly.com/in
For adverse events and safety reporting, please reach out to: mailbox_in-gps@lilly.com | For any additional information related to Lilly products, please reach out to: queries_in-medinfo@lilly.com.
This material (including any link) is intended solely for the use of the recipient(s) and may contain confidential information. Any unauthorized review, use, disclosure, copying, or distribution is strictly prohibited. If you are not the intended recipient, please notify the sender immediately and destroy all copies of the material. The information provided in this section is intended solely for the use of registered medical practitioner. This material is being provided to healthcare professionals only for their guidance and use. Nothing on this website/microsite/material should be construed as giving medical advice or making recommendations regarding any health-related decision or action.
© 2025 Eli Lilly and Company


