Dapacose (Dapagliflozin) is a Sodium-Glucose Cotransporter 2 (SGLT2) inhibitor drug, which is indicated in adults for the treatment of insufficiently controlled Type 2 Diabetes Mellitus as an adjunct to other medications as well as diet and exercise. Dapagliflozin is also used in adults for the treatment of symptomatic Chronic Heart Failure with reduced ejection fraction with or without Diabetes. The drug is also used for the treatment of Chronic Kidney Disease (CKD) patients upto Stage III (eGFR of greater or equal to 30ml/min/1.73m2.)
Dapacose is marketed by J.B. Chemicals & Pharmaceuticals Ltd. and available in two doses namely DAPACOSE 5mg and DAPACOSE 10mg.
Dapagliflozin inhibits the SGLT2 which is primarily located in the proximal tubule of the nephron. SGLT2 facilitates 90% of glucose reabsorption in the kidneys and so its inhibition allows for glucose to be excreted in the urine. This excretion allows for better glycemic control and potentially weight loss in patients with Type 2 Diabetes Mellitus.
Dapagliflozin is also used to reduce the risk of the need for hospitalization for Heart Failure in adults who have Type 2 Diabetes along with heart and blood vessel disease or who have multiple risk factors for developing heart and blood vessel disease. Recent evidence has also shown that the drug halts the progression of CKD in patients with, or without Diabetes.
2) To prevent the progression of Chronic Kidney Disease (CKD), Hospitalization for Heart Failure (hHF), MACE and CV death in patients with T2DM with CKD.
In Management of Type 2 Diabetes Mellitus, Dapagliflozin provides benefits beyond Glucose Control.
- a) Cardio protection
Dapagliflozin demonstrates reduction in the risk of CV death or worsening heart failure in patients with HFrEF, with & without diabetes. - b) Renal protection
Dapagliflozin delays initiaton of dialysis & reduces the number of deaths.
Dapa-HF2 (trial) demonstrated reduction in the risk of CV death or worsening heart failure in patients with HFrEF, with & without Diabetes.
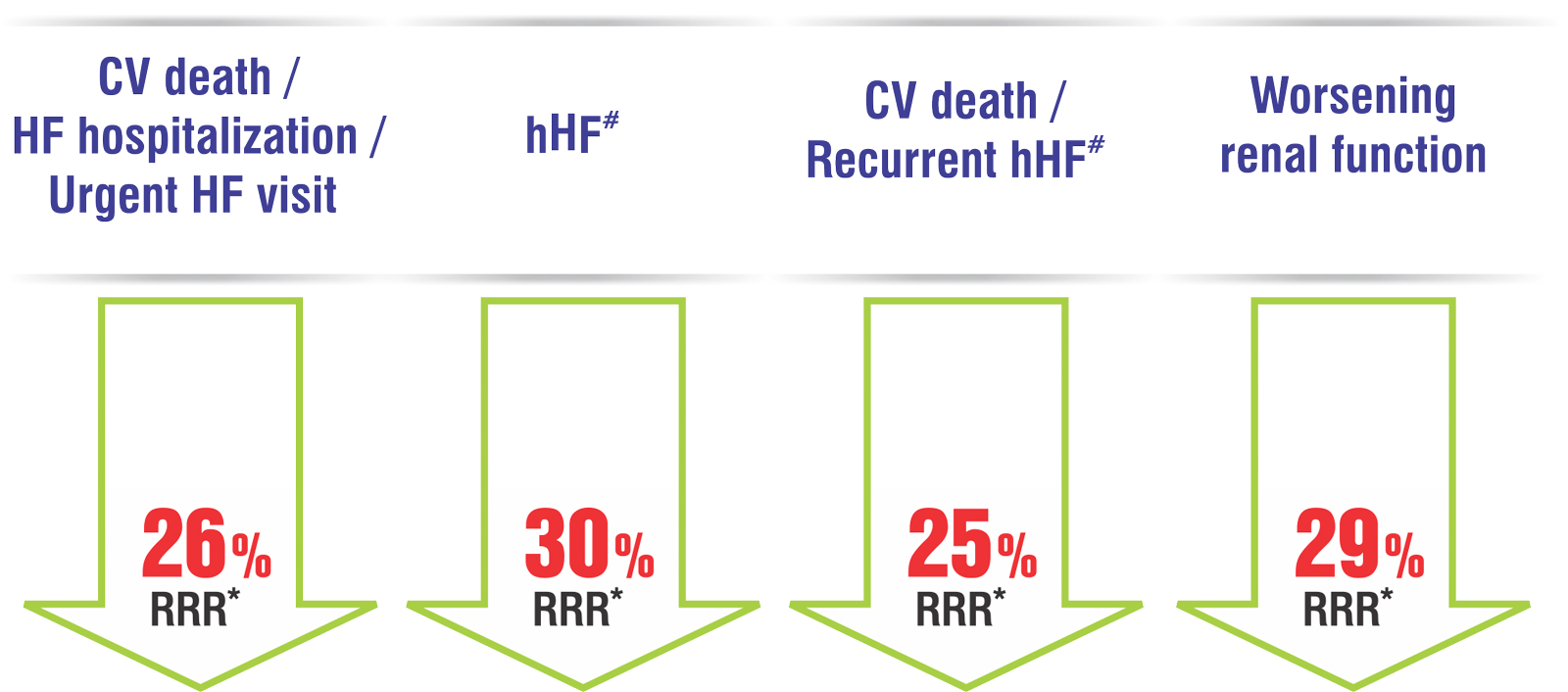
hHF#- Hospitalization for heart failure.
RRR*- Relative risk reduction.
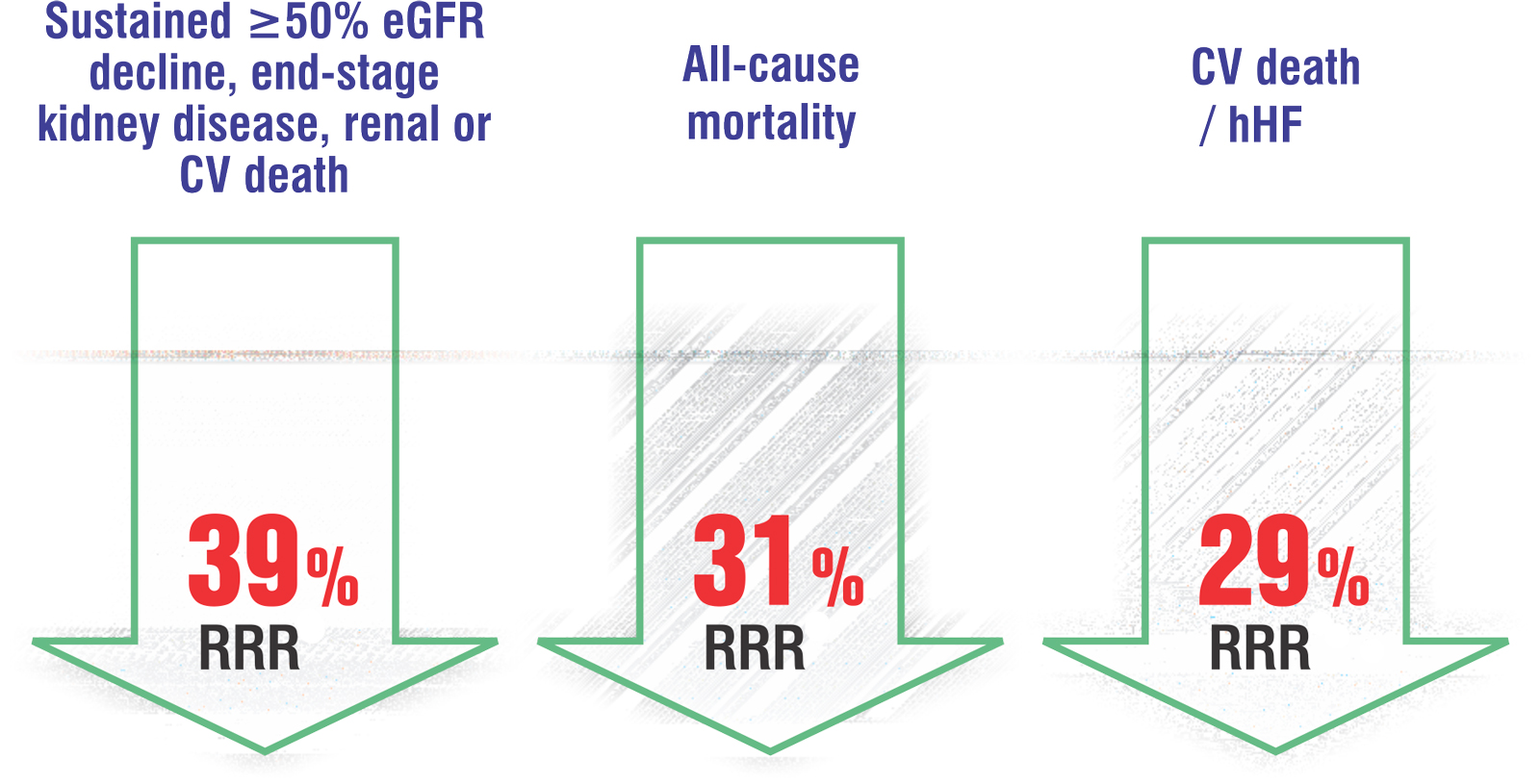
In patients with CKD3, with and without Type 2 Diabetes, Dapagliflozin significantly
- Reduces the risk of kidney failure.
- Reduces the risk of CV death or Heart Failure hospitalization.
- Prolongs survival.
Dapagliflozin is Approved for the treatment of CKD patients up to Stage III(eGFR>30ml/min/1.73m)
Featured Post
References
1. Das,et al. JACC,2020 sep 1;76 (9): 1117-1145,John B.Buse et al. Diabetologia (2020) 63:1667
2. Huang H et al, AM J Manag Care. 2018 Apr;24( 8 Suppl):S132-S137
3. Heerspink HJL et al. N Engl J Med 2020 oct 8;383( 15): 1436-1446,Recommendations of the SEC dated 8th Jan 2021
Type 2 Diabetes Mellitus
Dapagliflozin is indicated in adults for the treatment of insufficiently controlled Type 2 Diabetes Mellitus as an adjunct to diet and exercise.
- As monotherapy when metformin is considered inappropriate due to intolerance
- In addition to other medicinal products for the treatment of Type 2 Diabetes
Heart failure
Dapagliflozin is indicated in adults for the treatment of symptomatic Chronic Heart Failure with reduced ejection fraction.
CKD
Dapagliflozin is also indicated in Chronic Kidney Disease (CKD) patients upto Stage III (eGFR of greater or equal to 30ml/min/1.73m2.
Download the Product Leaflet,to see the details on Indications of Dapacose.
Dose
Type 2 Diabetes Mellitus
The recommended dose is 5mg or 10mg of Dapagliflozin once daily.When Dapagliflozin is used in combination with insulin or an insulin secretagogue, such as a sulphonylurea,a lower dose of insulin or insulin secretagogue may be considered to reduce the risk of hypoglycaemia.
Heart failure
The recommended dose is 10 mg Dapagliflozin once daily.In the DAPA-HF study, Dapagliflozin was administered in conjunction with other heart failure therapies.
Special populations:
Treatment of diabetes mellitus in patients with renal impairment
As glycaemic efficacy is dependent on renal function, Dapagliflozin should not be initiated to improve glycaemic control in patients with a glomerular filtration rate [GFR] < 60 mL/min and should be discontinued at GFR persistently below 45 mL/min.No dose adjustment is required based on renal function.
Treatment of heart failure in patients with renal impairment
No dose adjustment is required based on renal function.There is limited experience with dapagliflozin for the treatment of heart failure in patients with severe renal impairment (GFR < 30 mL/min).
Hepatic impairment
No dose adjustment is necessary for patients with mild or moderate hepatic impairment. In patients with severe hepatic impairment, a starting dose of 5 mg is recommended. If well tolerated, the dose may be increased to 10 mg.
Patients with Type 1 Diabetes Mellitus
Dapagliflozin 10 mg is not recommended for the treatment of in patients with Type 1 Diabetes Mellitus.
Elderly (≥ 65 years)
No dose adjustment is recommended based on age.
Paediatric population
The safety and efficacy of dapagliflozin in children aged 0 to < 18 years have not yet been established. No data are available.
Download the Product Leaflet,to see the details on Doses of Dapacose.
Method of administration
Dapagliflozin can be taken orally once daily at any time of day with or without food. Tablets are to be swallowed whole.
Download the Product Leaflet,to see the details on Method of Administration of Dapacose.
Hypersensitivity to the active substance or to any of the excipients listed.
Download the Product Leaflet,to see the details on Contraindications of Dapacose.
Download the Product Leaflet,to see the details on Warning and Precautions of Dapacose.
Download the Product Leaflet,to see the details on Drug Interactions of Dapacose.
Download the Product Leaflet,to see the details on Undesirable Side effects/ Overdose of Dapacose.
Download the Product Leaflet,to see the details on Pharmacodynamic and Pharmacokinetic Properties of Dapacose.