- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Nivolumab shows potential clinical activity for high-risk leukoplakia
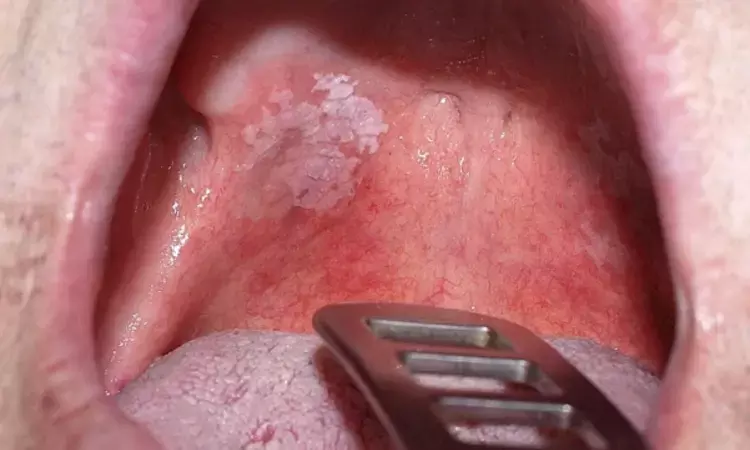
USA: An immune checkpoint therapy trial revealed that Nivolumab showed potential clinical activity for high-risk oral precancerous disease, adding that future trials should prioritize cancer-free survival endpoints and biomarker stratification. The study findings were published online in JAMA Oncology.
The phase 2 nonrandomized controlled trial treated 33 patients with high-risk oral proliferative verrucous leukoplakia with the programmed cell death 1 protein inhibitor nivolumab and showed variable lesion regression by degree and size of dysplasia in response to therapy, while 27% of patients developed invasive oral cancer after nivolumab.
"All whole-exome sequenced patients who progressed to develop cancer had 9p21.3 chromosomal loss," the study stated.
"We report the first (to our knowledge) nonrandomized clinical trial of immune checkpoint therapy (ICT) in patients with precancerous disease, specifically patients with high-risk oral precancer, to mitigate progression to oral squamous cell carcinoma," the researchers wrote.
Proliferative verrucous leukoplakia (PVL) is an aggressive oral precancerous disease with characteristics of a high risk of transformation to invasive oral squamous cell carcinoma (OSCC), and no therapies have been shown to impact its natural history. A recent study of the PVL immune landscape showed a cytotoxic T-cell–rich microenvironment, providing a strong rationale for determining immune checkpoint therapy.
Glenn J. Hanna, Dana-Farber Cancer Institute, Boston, Massachusetts, and colleagues aimed to determine the clinical activity and safety of anti–programmed cell death 1 protein (PD-1) therapy to treat high-risk PVL.
For this purpose, the researchers conducted a nonrandomized, open-label, phase 2 clinical trial from 2019 to 2021 at a single academic medical centre; with a follow-up at 21.1 months. Participants included a population-based sample of patients with PVL (multifocal, contiguous, or a single lesion ≥4 cm with any degree of dysplasia).
Patients underwent pretreatment biopsy (1-3 sites) and then received 4 nivolumab doses (480 mg intravenously) every 28 days, followed by rebiopsy and intraoral photographs at each visit.
The primary endpoint was the change in composite score (degree and size of dysplasia) from before to after treatment (major response [MR]: >80% decrease in score; partial response: 40%-80% decrease). Secondary analyses were immune-related adverse events, PD-1 ligand 1 (PD-L1) expression, cancer-free survival (CFS), 9p21.3 deletion, and other exploratory genomic and immunologic associations of response.
The study enrolled 33 patients (median age, 63 years; 55% were female), including 24% with previously resected early-stage oral squamous cell carcinoma.
The study revealed the following findings:
- 36% of patients had a response by composite score (9% MRs), and 4 had progressive disease (>10% composite score increase, or cancer).
- 27% of patients developed OSCC during the trial, with a 2-year CFS of 73%.
- Because of toxic effects, 6% of patients discontinued; 21% experienced grade 3 to 4 immune-related adverse events.
- PD-L1 combined positive scores were not associated with response or CFS.
- Of 20 whole-exome sequenced patients, all 6 patients who had progression to OSCC after nivolumab treatment exhibited 9p21.3 somatic copy-number loss on pretreatment biopsy, while only 29% of patients who did not develop OSCC had 9p21.3 loss.
"This immune checkpoint therapy precancer nonrandomized clinical trial met its prespecified response endpoint, suggesting potential clinical activity for nivolumab in high-risk PVL," the researchers wrote.
"Findings identified immunogenomic associations to inform future trials in this precancerous disease with an unmet medical need that has been difficult to study," they concluded.
Reference:
Hanna GJ, Villa A, Nandi SP, et al. Nivolumab for Patients With High-Risk Oral Leukoplakia: A Nonrandomized Controlled Trial. JAMA Oncol. 2024;10(1):32–41. doi:10.1001/jamaoncol.2023.4853
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


