- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Amenamevir safe and effective treatment option for patients with herpes zoster
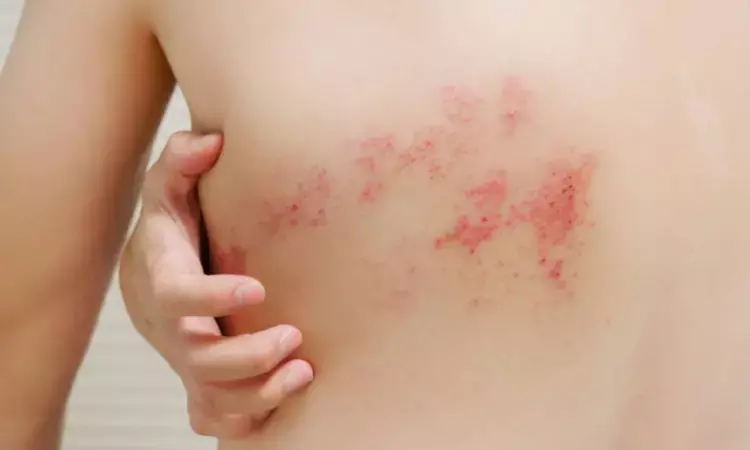
Japan: A recent study published in The Journal of Dermatology has shown helicase - primase inhibitor amenamevir (AMNV) to be a safe and effective treatment option in patients with herpes zoster in a real-world clinical setting. Amenamevir showed a favourable safety profile and high cutaneous improvement rates.
In the REWARD study, including 3098 Japanese patients with herpes zoster, the researchers found that amenamevir, a helicase–primase inhibitor, demonstrated high efficacy, with an overall improvement rate of 95.5%. Longer treatment duration, lower pain levels, and less severe cutaneous lesions were associated with higher improvement rates.
Amenamevir was approved for herpes zoster in Japan in 2017. Shinich Imafuku, Department of Dermatology, Fukuoka University, Fukuoka, Japan, and colleagues conducted a 1-month postmarketing observational study to assess the real-world efficacy (pain resolution and cutaneous improvement) and safety of AMNV in patients with herpes zoster.
Of the 3453 patients registered between 2018 and 2020, 3110 were included in the safety analyses. The mean age was 63.7 ± 17.5 years, and 57.9% of patients were aged ≥65 years. Most patients had moderate (41.0%) or mild (53.3%) cutaneous lesions.
The authors reported the following findings:
- 43.9%, 25.6%, and 12.5% of patients had pain at the levels of 1–3, 4–6, and 7–10 on the numerical rating scale.
- 30.0%, 27.2%, and 16.1% of patients were concomitantly treated with analgesics: acetaminophen, nonsteroidal anti-inflammatory drugs, and Ca2+ channel α 2δ ligands, respectively, and 10.6% were treated with topical antiherpetic drugs.
- Adverse drug reactions occurred in 0.77% of patients, including four serious adverse drug reactions in four patients (thrombocytopenia, hyponatremia, rhabdomyolysis, and rash).
- Regarding important potential risks, cardiovascular events, renal disorder, and decreased platelets were observed in one, one, and two patients, respectively.
- The cutaneous improvement rate (significantly improved or improved) was 95.5%, with significantly higher improvement rates in patients treated with amenamevir for 7 days and in patients with less severe cutaneous lesions or less pain.
- Factors affecting the time to pain resolution were the severity of cutaneous lesions and pain at the start of amenamevir treatment and older age.
"Our study showed Amenamevir to be a safe and effective treatment option for patients with herpes zoster, with a favourable safety profile and high cutaneous improvement rates," the researchers concluded.
Reference:
Imafuku, S., Korematsu, K., Mori, N., Kani, T., & Matsui, K. Real-world safety and efficacy of amenamevir in patients with herpes zoster in Japan: A postmarketing observational study (REWARD). The Journal of Dermatology. https://doi.org/10.1111/1346-8138.16876
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


