- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Baricitinib not linked to increased VTE Risk in Alopecia Areata Patients: Study
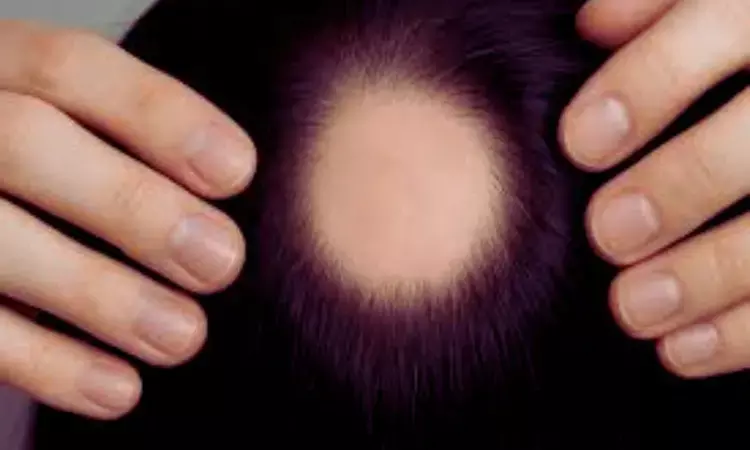
A new study published in the International Journal of Dermatology revealed that alopecia areata patients treated with baricitinib had no reported thrombotic events over 6 months, indicating no increased risk of venous thromboembolism (VTE) in this population.
Based on post-marketing data from patients with rheumatoid arthritis (RA) receiving tofacitinib, the European Medicines Agency (EMA) released an advisory in January 2023 on the risk of VTE associated with Janus kinase inhibitors (JAKi) in chronic inflammatory disorders. Given that RA is a recognized independent risk factor for VTE, this might be biased.
Additionally, the effects of JAKi differ due to differences in enzyme selectivity. In this investigation, VTE risk factors were examined in a cohort of AA patients who were eligible for baricitinib therapy. Additionally, any changes in laboratory VTE markers following 6 months of baricitinib treatment were evaluated.
The patients with moderate-to-severe AA who were ≥ 18 years old, suitable for therapy with baricitinib 4 mg, and who presented to the department consecutively between July 2023 and July 2024 were included in a prospective observational research. During the screening appointment, baseline clinical and demographic information was gathered.
Patients with major (a prior VTE episode) or minor (smoking status, active malignancies, family history of VTE, prior thrombophlebitis, recent trauma or fractures, prolonged immobilization, major recent surgeries, use of estrogen-progestin contraceptives, and recurrent miscarriages) clinical risk factors for VTE were also identified through a comprehensive medical history check. Additionally, baseline and 6-month therapy levels were assessed for antiphospholipid antibodies (lupus anticoagulant, anti-cardiolipin, and anti-β2 glycoprotein I), protein C, antithrombin III, homocysteine, protein S, and factor VIII.
After being evaluated for baricitinib therapy eligibility, 47 individuals with moderate-to-severe AA were included to the trial. Based on the previously published criteria, none of the screened patients showed a contraindication to the therapy. In terms of clinical VTE risk factors at baseline, 1 patient (2.1%) had a history of superficial thrombophlebitis, 5 women (10.6%) were taking combination estrogen-progestin contraceptives, and 8 out of 47 patients (17%) were heavy smokers.
There were no significant risk factors in any of the patients. Although two patients (4.2%) had bone fractures, which are recognized clinical risk factors for VTE, there were no thrombotic events while on baricitinib. Laboratory tests conducted on 37 out of 47 patients after 6 months of continuous medication revealed no changes in the majority of patients. Overall, these results imply that the risk of experiencing adverse events from VTE might be decreased for individuals who are eligible for baricitinib therapy.
Source:
Caldarola, G., Ferretti, A., Pinto, L. M., Di Gennaro, L., De Luca, E., Falco, G. M., De Simone, C., De Cristofaro, R., & Peris, K. (2025). No increased risk of thromboembolic events during 6-month treatment with baricitinib in patients with alopecia areata. International Journal of Dermatology, ijd.70051. https://doi.org/10.1111/ijd.70051
Neuroscience Masters graduate
Jacinthlyn Sylvia, a Neuroscience Master's graduate from Chennai has worked extensively in deciphering the neurobiology of cognition and motor control in aging. She also has spread-out exposure to Neurosurgery from her Bachelor’s. She is currently involved in active Neuro-Oncology research. She is an upcoming neuroscientist with a fiery passion for writing. Her news cover at Medical Dialogues feature recent discoveries and updates from the healthcare and biomedical research fields. She can be reached at editorial@medicaldialogues.in
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


