- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Berdazimer gel 10.3 % found effective in clearing molluscum lesions in phase 3 SIMPLE clinical trial
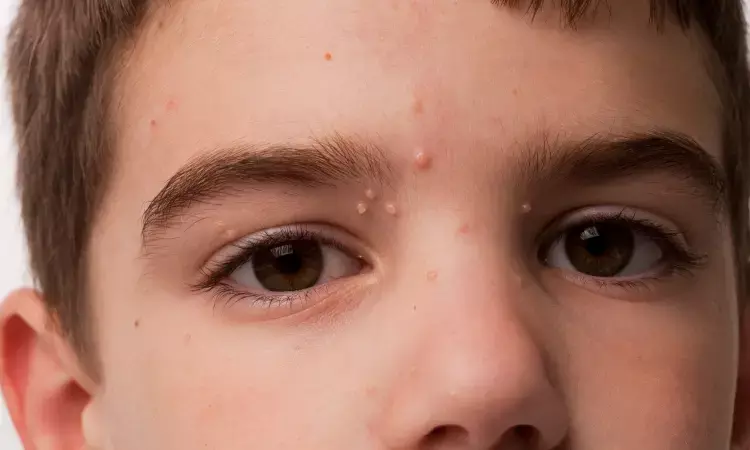
United States: According to findings from phase 3 B-SIMPLE clinical trials, new data presented during the annual meeting of the American Academy of Dermatology (AAD) 2023, berdazimer gel 10.3 % will be the first potential drug by the US Food and Drug Administration (FDA) for molluscum in January 2024
It is already known that Berdazimer gel 10.3% is a topical nitric oxide for the management of molluscum contagiosum, a highly contagious viral infection of the skin in Phase 3 development.
Researchers have assessed the safety of berdazimer gel 10.3% in three phase 3 molluscum studies B-SIMPLE 1, 2 and 4.
Trial B-SIMPLE-1, 2 and 4 were randomized double-blind and evaluated the safety and efficacy of the application of berdazimer gel 10.3% once daily or vehicle gel for 12 weeks.
The researchers have included children of ≥6 months of age with a history of 3-70 molluscum lesions. They set Safety endpoints as the incidence of treatment-emergent adverse events (TEAEs) and local skin reactions (LSRs).
The study results could be summarised as follows:
- A total of 1596 patients were enrolled with a mean age of 6.7 years.
- 50.6% of patients were male
- The mean lesion count was 20.2, and the n value of 916:680 berdazimer:vehicle
- Patients with ≥1 TEAE were shown as 46.7% (428) vs 24.9% (169); most were mild 24.0% (220) vs 17.4% (118) or moderate 21.0% (192) vs 6.9% (47) in severity.
- The most frequent TEAEs were application site pain, erythema, and pruritus, constituting 18.7% vs 4.9%, 11.9% vs 1.6% and 5.7% vs 1.0%.
- TEAEs leading to study drug discontinuation were few: 4.6% (42) vs 0.7% (5).
- TEAEs related to the study drug: 36.7% (336) vs 11.9% (81); none were serious.
- LSRs were more frequent in berdazimer with an LSR score ≥1 of 66.9% vs 40.7%
The main author, John C. Browning, said that, “This gel provides complete lesion clearance in 30.0% of all treated patients by week 12, versus just 19.8% of patients receiving vehicle with Odds ratio of 1.75. The complete clearance rate in children aged 6 to <12 years is 31.1% complete with berdazimer gel, versus just 14.0% among the pediatric vehicle arm.”
The overall safety and tolerability of berdazimer gel 10.3% was demonstrated in B-SIMPLE Phase 3 program.
Further reading:
Safety of Berdazimer Gel 10.3% in the Treatment of Molluscum Contagiosum: Integrated Results from the B-SIMPLE Phase 3 Program
BDS, MDS in Periodontics and Implantology
Dr. Aditi Yadav is a BDS, MDS in Periodontics and Implantology. She has a clinical experience of 5 years as a laser dental surgeon. She also has a Diploma in clinical research and pharmacovigilance and is a Certified data scientist. She is currently working as a content developer in e-health services. Dr. Yadav has a keen interest in Medical Journalism and is actively involved in Medical Research writing.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


