- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Cendakimab Shows Efficacy and Safety in Treating Moderate to Severe Atopic Dermatitis: Results from Clinical Trial
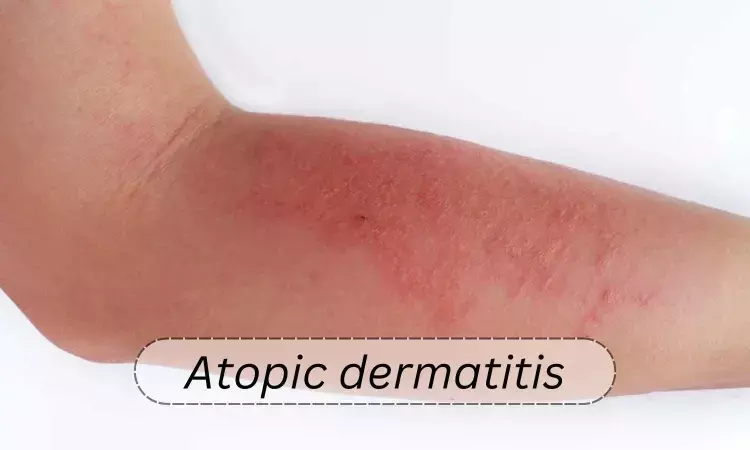
USA: In a phase 2 randomized clinical trial of 221 patients with moderate to severe atopic dermatitis (AD), treatment with cendakimab, an investigational anti-interleukin (IL)-13 monoclonal antibody, demonstrated efficacy after 16 weeks.
The findings, published in JAMA Dermatology, demonstrated improvements in pruritus, skin clearance, and the extent and severity of AD compared with placebo. Cendakimab was generally safe and well-tolerated.
Cendakimab selectively targets interleukin (IL)-13, a type 2 cytokine involved in the pathogenesis of atopic dermatitis (AD), by blocking its binding to IL13R-α1 and IL13R-α2 receptors. Proof-of-concept studies in AD endorse cendakimab as a potential treatment for type 2 inflammatory diseases. Considering this, Andrew Blauvelt, Oregon Medical Research Center, Portland, Oregon, and colleagues aimed to evaluate the safety and efficacy of cendakimab compared with placebo in patients with moderate to severe AD.
For this purpose, the researchers conducted a phase 2, randomized, double-blind, placebo-controlled, parallel-group, dose-ranging clinical trial from 2021 to 2021. It enrolled adult patients with moderate to severe AD and inadequate response to topical medications at 69 sites in 5 countries (Japan [n = 17], US [n = 26], Czech Republic [n = 8], Poland [n = 9], and Canada [n = 9]).
Patients were randomized in a 1:1:1:1 ratio to receive subcutaneous cendakimab, 360 mg, every two weeks; 720 mg, every two weeks; 720 mg, once weekly; or placebo.
The study evaluated the mean percentage change in Eczema Area and Severity Index (EASI) scores from baseline to week 16. Hierarchical testing with adjustment for multiple comparisons was conducted for the following comparisons: 720 mg once weekly versus placebo, followed by 720 mg every two weeks vs placebo, and finally, 360 mg every two weeks vs placebo.
The study led to the following findings:
- Two-hundred twenty-one patients were randomized, and 220 received the study drug (43% women; mean age, 37.7 years; 720 mg, once weekly [24%]; 720 mg, every two weeks [25%]; 360 mg, every two weeks [25%]; placebo [26%]).
- The primary efficacy endpoint was met for cendakimab, 720 mg, once weekly versus placebo (–84.4 versus –62.7) but missed statistical significance for 720 mg, every two weeks (–76.0 versus –62.7).
- The treatment effect for 360 mg every two weeks (−16.3 versus placebo) was comparable with 720 mg once weekly (−21.8); however, significance was not claimed because the hierarchical testing sequence was interrupted.
- Of patients with treatment-emergent adverse events leading to discontinuation, 7.4% received 720 mg once weekly; 3.6% 720 mg every two weeks; 1.8% 360 mg every two weeks; and 3.6% placebo.
"In the randomized clinical trial, cendakimab proved effective, safe, and well-tolerated for patients with moderate to severe atopic dermatitis (AD). The primary endpoint, a significant reduction in Eczema Area and Severity Index (EASI) scores at week 16, was achieved with the 720 mg once-weekly dose. Across all doses, cendakimab showed continuous improvement in AD over the 16-week treatment period," the researchers concluded.
Reference:
Blauvelt A, Guttman-Yassky E, Lynde C, et al. Cendakimab in Patients With Moderate to Severe Atopic Dermatitis: A Randomized Clinical Trial. JAMA Dermatol. Published online July 17, 2024. doi:10.1001/jamadermatol.2024.2131
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


