- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Crisaborole may improve signs and symptoms of Stasis Dermatitis,reveals study
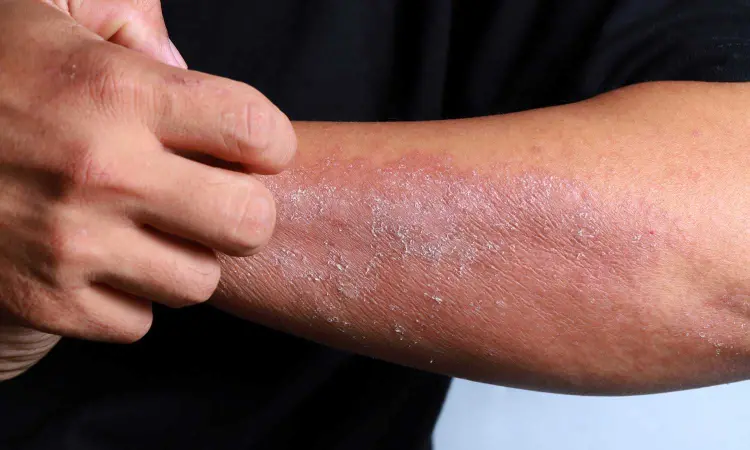
Stasis Dermatitis (SD) is often associated with poor venous circulation and can lead to complications such as skin ulcers and infection. Treatment options for SD are limited, and there is a need for effective and well-tolerated therapies. Crisaborole's anti-inflammatory properties make it a potential candidate for the management of SD.
Stasis dermatitis (SD) is a common inflammatory skin condition affecting individuals aged 45 years and older, characterized by itching, redness, and swelling. Crisaborole ointment, 2%, a nonsteroidal topical phosphodiesterase 4 inhibitor, has been approved for the treatment of mild-to-moderate atopic dermatitis.
A recent phase 2a study aimed to assess the efficacy and safety of crisaborole in patients with SD. This study was published in the Journal Of The American Academy Of Dermatology by Jonathan I. and colleagues. The randomized, double-blind, vehicle-controlled study enrolled 65 participants with SD without active ulceration. Patients received either crisaborole or vehicle ointment twice daily for 6 weeks. The primary endpoint was the percentage change from baseline in total sign score at week 6, assessed by in-person and central reader evaluations.
The key findings of the study were:
Crisaborole-treated participants showed a significant reduction in total sign score from baseline compared to the vehicle group.
In-person assessment by nondermatologists demonstrated a reduction of 32.4% in the crisaborole group versus 18.1% in the vehicle group (P = .0299).
Central reader assessment of photographs showed an even greater reduction of 52.5% versus 10.3% (P = .0004).
Efficacy outcomes, including success and improvement per Investigator's Global Assessment score and lesional percentage body surface area, reached statistical significance based on central reader assessments but not in-person evaluations.
Skin and subcutaneous tissue disorders were the most common treatment-emergent adverse events with crisaborole, indicating that the treatment was generally well tolerated.
Crisaborole ointment, 2%, demonstrated efficacy in improving signs and symptoms of SD in patients aged 45 years and older. The study findings support the use of crisaborole as a potential treatment option for SD. Central reader assessment showed promise as a method for evaluating treatment efficacy in decentralized clinical research settings.
Reference:
Silverberg, J. I., Kirsner, R. S., Margolis, D. J., Tharp, M., Myers, D. E., Annis, K., Graham, D., Zang, C., Vlahos, B. L., & Sanders, P. Efficacy and safety of crisaborole ointment, 2%, in participants aged ≥45 years with stasis dermatitis: Results from a fully decentralized, randomized, proof-of-concept phase 2a study. Journal of the American Academy of Dermatology,2024. https://doi.org/10.1016/j.jaad.2023.12.048
Dr Riya Dave has completed dentistry from Gujarat University in 2022. She is a dentist and accomplished medical and scientific writer known for her commitment to bridging the gap between clinical expertise and accessible healthcare information. She has been actively involved in writing blogs related to health and wellness.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


