- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
FDA approved Bimzelx for plaque psoriasis now commercially available
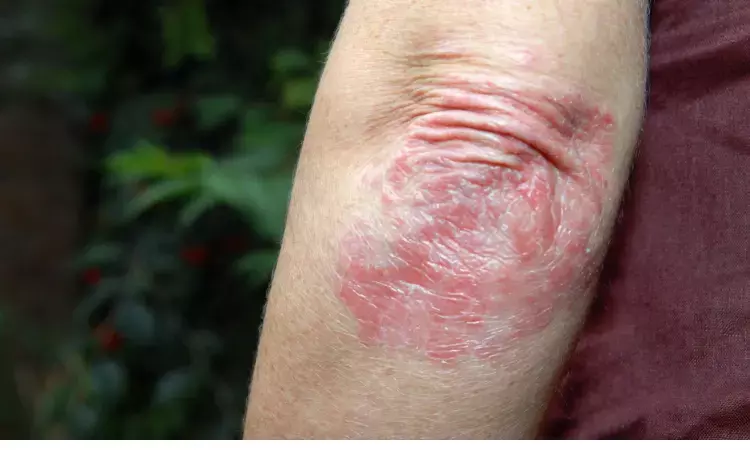
In October US Food and Drug Administration had approved for plaque psoriasis. Bimzelx, an IL-17A and IL-17F inhibitor is now commercially available for adult patients of psoriasis who are candidates for systemic or phototherapy as autoinjector.
BIMZELX is available as an autoinjector and a pre-filled syringe. BIMZELX may be administered subcutaneously by a healthcare professional, or a patient may self-inject after proper training. People living with moderate-to-severe plaque psoriasis should talk to their healthcare provider to see if BIMZELX may be right for them.
“While the treatment landscape for psoriasis has evolved in recent years, many unmet needs remain for the more than 7.5 million adults in the U.S. living with the disease,” said Dr. Jeffrey Stark, Head of Medical, U.S. Immunology, UCB. “With BIMZELX now available, patients and their healthcare providers have access to a treatment that has proven to consistently deliver rapid, complete, and maintained skin clearance from the first dose.”
BIMZELX is the first and only approved psoriasis treatment designed to selectively inhibit two key cytokines driving inflammatory processes – interleukin 17A (IL-17A) and interleukin 17F (IL-17F). The approval of BIMZELX is supported by data from three Phase 3, multicenter, randomized, placebo and/or active comparator-controlled trials (BE READY, BE VIVID, and BE SURE), which evaluated the efficacy and safety of BIMZELX in 1,480 adults with moderate-to-severe plaque psoriasis.
“At UCB, we are committed to creating accessible treatments that empower people living with psoriasis to achieve their own goals and live their best possible lives,” said Camille Lee, Head of U.S. Immunology, UCB. “That’s why we developed BIMZELX Navigate™, a tailored patient support program designed to help patients with access, affordability, and treatment support throughout their treatment journeys with BIMZELX.”
Through BIMZELX Navigate, UCB offers tailored patient support to all those with moderate-to-severe plaque psoriasis upon receiving their BIMZELX prescription. Upon enrollment, patients will be paired with a dedicated Nurse Navigator. This licensed Registered Nurse will be available to discuss treatment goals, train patients on how to administer BIMZELX, connect patients to Co-Pay support, and keep patients up-to-date about their BIMZELX shipment and insurance status. Eligible patients living in the U.S. may enroll in BIMZELX Navigate at https://www.bimzelx.com/patient-support.
UCB is actively engaging in access conversations with payers, with a focus on our patient value strategy and keeping the patient at the center of these discussions. Based on these conversations, we are progressing well toward our goal to enable affordable access to our medicines for all people who need them, in a way that is sustainable for patients, society and UCB. While we work toward securing access for patients with payer organizations, our BIMZELX Navigate program is in place and successfully operating to get patients started. Full affordability information can be found at ucb-usa.com/Sustainability/Affordability/Bimzelx-Pricing-Info and http://www.BIMZELX.com. For additional medical information, patient assistance or any other information, please call UCBCares® at 1-844-599-CARE (2273) or visit askucbcares.com.
Key Findings from the Phase 3 Clinical Development Program
The efficacy and safety of BIMZELX were evaluated in three Phase 3 studies, versus placebo and ustekinumab (BE VIVID), versus placebo (BE READY), and versus adalimumab (BE SURE). All studies met their co-primary endpoints and all ranked secondary endpoints.
Patients treated with BIMZELX achieved superior levels of skin clearance at week 16, compared to those who received ustekinumab (ranked secondary endpoint, BE VIVID; p<0.0001), placebo (co-primary endpoint, BE READY and BE VIVID; p<0.0001) and adalimumab (co-primary endpoint, BE SURE; p<0.001), as measured by at least a 90 percent improvement in the Psoriasis Area & Severity Index (PASI 90) and an Investigator's Global Assessment (IGA) response of clear or almost clear skin (IGA 0/1). Ranked secondary endpoints included PASI 75 at week 4 and PASI 100 (complete skin clearance) at week 16. Key findings across all studies include:
- Clear or Almost Clear Skin: More than eight out of 10 patients receiving BIMZELX (320 mg every four weeks [Q4W]) achieved PASI 90 and IGA 0/1 at week 16.
- Complete Skin Clearance: Approximately six out of 10 patients receiving BIMZELX (320 mg Q4W) achieved PASI 100 at week 16.
- Speed of Response: Clinical responses achieved with BIMZELX were rapid, with more than seven out of 10 patients achieving PASI 75 at week 4 following one dose (320 mg).
- Maintenance of Response: Clinical responses achieved with BIMZELX at week 16 (PASI 90 and PASI 100) were maintained for up to one year. Long-term data showed that clinical responses were maintained in the vast majority of patients through to three years of BIMZELX treatment.
The most common adverse reactions (≥ 1%) are upper respiratory infections, oral candidiasis, headache, injection site reactions, tinea infections, gastroenteritis, Herpes Simplex Infections, acne, folliculitis, other Candida infections, and fatigue.
About BIMZELX (bimekizumab-bkzx)
Bimekizumab is a humanized IgG1 monoclonal antibody that selectively binds to IL-17A, IL-17F and IL-17AF cytokines, blocking their interaction with the IL-17RA/IL-17RC receptor complex. Elevated levels of IL-17A and IL-17F are found in lesional psoriatic skin.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


