- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
FDA approves dupilumab for treatment of atopic dermatitis in pediatric patients
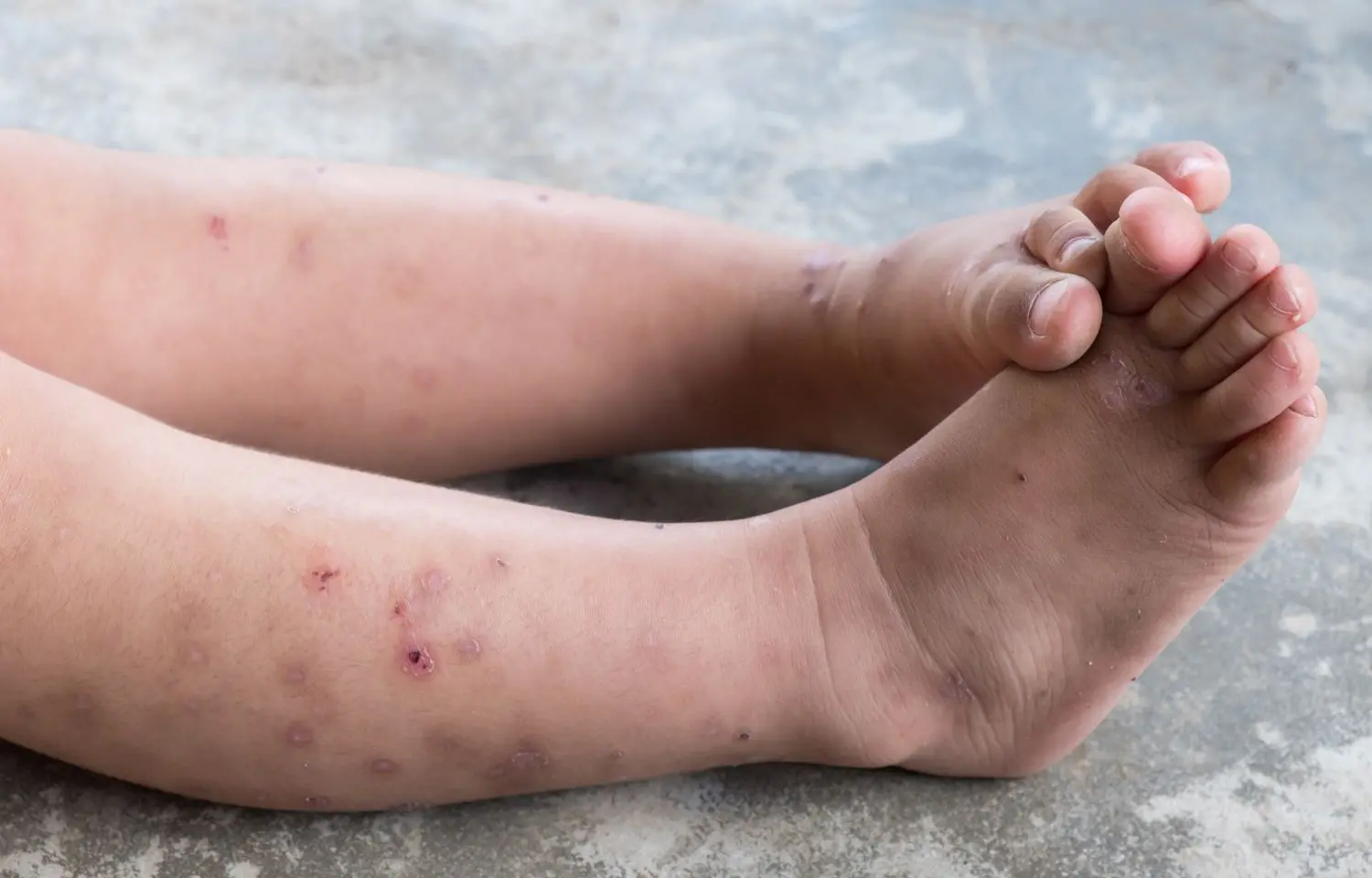
TARRYTOWN, N.Y. and PARIS: The U.S. Food and Drug Administration (FDA) has approved Dupixent® (dupilumab) for children aged 6 months to 5 years with moderate-to-severe atopic dermatitis whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable. Regulatory filings for this age group are underway by the European Medicines Agency and regulatory authorities in additional countries.
"Moderate-to-severe atopic dermatitis in babies and young children is more than just a rash-the intense itch can make them scratch uncontrollably throughout the day and night and cause their skin to crack and bleed," said Julie Block, President and Chief Executive Officer at National Eczema Association. "Caregivers do their best to manage skincare routines multiple times a day, but for many, topical treatments are not enough. We're pleased to see how scientific innovation and research continues to address unmet needs for the atopic dermatitis community, and we're hopeful for the positive impact Dupixent can have for these children and their families."
Atopic dermatitis is a chronic type 2 inflammatory skin disease. Eighty-five to ninety percent of patients first develop symptoms before 5 years of age, which can often continue through adulthood. Symptoms include intense, persistent itch and skin lesions that cover much of the body, resulting in skin dryness, cracking, pain, redness or darkening, and crusting and oozing. In the U.S., more than 75,000 children aged 5 years and younger have uncontrolled moderate-to-severe disease and are most in need of new treatment options. Moderate-to-severe atopic dermatitis may also significantly impact the quality of life of a young child and their caregivers.
"Young children with moderate-to-severe atopic dermatitis are a significantly underserved population of patients, who spend vulnerable years of their lives suffering through the relentless and far-reaching effects of this chronic disease," said George D. Yancopoulos, M.D., Ph.D., President and Chief Scientific Officer at Regeneron, and a principal inventor of Dupixent.
"Dupixent has changed the atopic dermatitis treatment paradigm-significantly clearing skin and reducing itch-by targeting an underlying cause of this disease without broadly suppressing the immune system. Today's approval brings the proven efficacy and, importantly, well-established safety profile of Dupixent to these young children, making it the first of its kind to be approved for any U.S. patient aged six months or older living with this debilitating disease."
"Until today, treatment options in the U.S. for infants and children under the age of 6 suffering from moderate-to-severe atopic dermatitis have been limited to topical steroids-which may be associated with significant safety risks when used long-term. This has left patients and their caregivers in desperate need of medicines that can better address the chronic, long-term nature of the disease," Naimish Patel, M.D, Senior Vice President, Head of Global Development, Immunology and Inflammation at Sanofi.
"These young people, and their families, often struggle to cope with the significant impact itch can have on them. This approval means that Dupixent, with its well-established safety and efficacy profile, is now available to some of the youngest people living with this disease."
The FDA evaluated Dupixent under Priority Review, which is reserved for medicines that represent potentially significant improvements in efficacy or safety in treating serious conditions. The approval is based on data that include a Phase 3 trial evaluating Dupixent every four weeks (200 mg or 300 mg, based on body weight) plus low-potency topical corticosteroids (TCS) or TCS alone. The trial met the primary and all secondary endpoints. At 16 weeks, patients who received Dupixent with TCS experienced the following, compared to TCS alone (placebo):
- 28% achieved clear or almost-clear skin compared to 4% with placebo, the primary endpoint.
- 53% achieved 75% or greater improvement in overall disease severity from baseline compared to 11% with placebo, the co-primary endpoint outside of the U.S.
- 48% achieved clinically meaningful reduction in itch compared to 9% with placebo.
The safety profile of Dupixent observed through 16 weeks in children aged 6 months to 5 years was similar to the safety profile in patients 6 years and older with atopic dermatitis. The long-term safety profile of Dupixent in children aged 6 months to 5 years through 52 weeks was also similar to the safety profile observed in the pivotal trial and consistent with what was observed in older patients with atopic dermatitis. Hand-foot-and-mouth disease and skin papilloma were, respectively, reported in 5% and 2% of Dupixent patients aged 6 months to 5 years, and none of these cases led to treatment discontinuation.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


