- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
FDA Approves tapinarof cream- first nonsteroidal treatment for plaque psoriasis
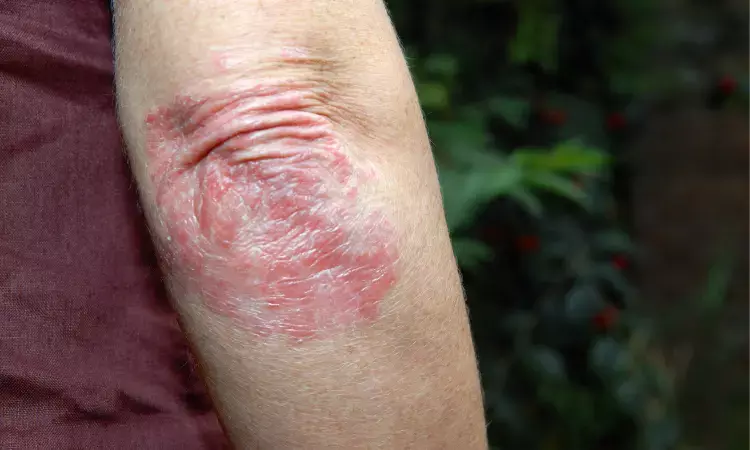
The Food and Drugs Administration has approved Vtama cream for treating plaque psoriasis with varying severity. The approval was announced by pharmaceutical company Dermavant Sciences.
Tapinarof cream, 1%, is an aryl hydrocarbon receptor agonist, indicated for the topical treatment of plaque psoriasis in adults. This approval makes VTAMA cream the first and only FDA-approved steroid-free topical medication in its class.
"We are delighted with our FDA-approved label for VTAMA cream, which is for adults with psoriasis, regardless of disease severity, and with an unlimited duration of use. In anticipation of today's approval, we have a fully built commercial infrastructure in place, and I am excited to say we will have product in the channel in the first week of June. As the first and only approved drug in its class in the U.S., the FDA's approval of VTAMA cream provides an effective new non-steroidal treatment option for millions of adults living with plaque psoriasis and represents a major milestone for Dermavant and its stakeholders," said Todd Zavodnick, Chief Executive Officer of Dermavant.
"At Dermavant, we are committed to advancing novel, patient-focused innovation in immuno-dermatology. As such, we are proud to have developed a topical treatment in VTAMA cream that provides not only efficacy over 52 weeks but can also be used on all body areas, including on sensitive locations, such as face, skin folds, neck, genitalia, anal crux, inflammatory areas, and axillae. In addition, an approximately four month off-treatment remittive effect (median time to first worsening), leads us to believe that VTAMA cream has the potential to become the preferred topical option for this chronically underserved patient population and among the physicians who treat them."
"Following 20-plus years of minimal innovation in the topical psoriasis treatment space1,2,3,4, I believe the approval of VTAMA cream is an important step in establishing a new treatment option for adults with mild, moderate and severe plaque psoriasis5," said Mark Lebwohl, MD, FAAD, Dean for Clinical Therapeutics and Waldman Professor and Chairman Emeritus of the Kimberly and Eric J. Waldman Department of Dermatology, Icahn School of Medicine at Mount Sinai in New York and lead author of the Phase 3 studies of VTAMA cream published in The New England Journal of Medicine.
"As a clinician, I'm excited to finally have a versatile, once-daily, steroid-free topical treatment that is backed by extensive clinical trial data supporting its favorable safety and efficacy profile and a demonstrated remittive effect of approximately four months in patients off therapy."
Across PSOARING 1 and PSOARING 2, VTAMA cream demonstrated highly statistically significant improvement in Physician Global Assessment (PGA)6 score of "clear" (PGA=0) or "almost clear" (PGA=1) with a minimum 2-grade improvement compared with vehicle from baseline at week 12. VTAMA cream also demonstrated a highly statistically significant improvement in all secondary endpoints versus vehicle, including ≥75% Improvement in Psoriasis Area and Severity Index (PASI) score (PASI-75) from baseline at week 127. The adverse event (AE) profile of VTAMA cream reported in both PSOARING 1 and PSOARING 2 demonstrated that the majority of AEs were localized to the site of application and were mild to moderate in nature. The most common AEs of subjects treated with VTAMA cream were folliculitis, nasopharyngitis, and contact dermatitis.
Eligible patients who completed PSOARING 1 or PSOARING 2 could enroll in PSOARING 3, a Phase 3 Long Term Extension (LTE) study, which consisted of an additional 40 weeks of open-label treatment with VTAMA cream, followed by a four-week follow-up. As such, patients who were randomized to VTAMA cream in PSOARING 1 or PSOARING 2 and who also completed the Phase 3 LTE study received VTAMA cream treatment for up to 52 weeks. 92% of patients who completed PSOARING 1 and PSOARING 2 enrolled in the Phase 3 LTE study.
Over 40% of Phase 3 LTE study patients (n=312/763) achieved complete disease clearance (PGA=0) at least once during the study period. For patients randomized to VTAMA cream in PSOARING 1 and PSOARING 2 who achieved a PGA of 0 during the 12-week study and subsequently enrolled in the Phase 3 LTE study (n=73), VTAMA cream demonstrated a remittive effect (maintenance of PGA of 0 or 1 while off therapy) with a median duration to first worsening of approximately four months. Among a larger cohort of patients who either entered the Phase 3 LTE study with a PGA score of 0 or achieved one during the LTE study (n=312), the mean duration of remittive effect off-therapy was 130 days.
In the Phase 3 LTE study, VTAMA cream demonstrated safety and tolerability consistent with PSOARING 1 and PSOARING 2. Treatment emergent adverse events were mostly mild to moderate in nature and restricted to application sites.
Responses to a questionnaire, which were assessed at Phase 3 LTE study completion (week 40 or early termination), demonstrated consistent high rates of satisfaction across all evaluated parameters. Of the 78.5% (n=599) of patients from Phase 3 LTE study who completed the survey: 85.8% of patients felt they could easily manage their psoriasis with VTAMA cream; 83.6% were satisfied with how well VTAMA cream worked; 81.7% considered it more effective than prior topical treatments, and most patients strongly agreed or agreed VTAMA cream absorbed quickly (89.5%), was not greasy (89.0%), and felt good on their skin (79.9%).
"We believe VTAMA cream has the potential to make a meaningful difference in the lives of patients with plaque psoriasis as well as their healthcare providers," said Philip M. Brown, M.D., J.D., Chief Medical Officer of Dermavant. "We are continuing to leverage our experience and insights with the active ingredient, tapinarof, and potentially other AhR molecules, for potential application to other conditions in dermatology and additional inflammatory and immunology indications."
In September 2021, Dermavant Sciences announced that it dosed its first patient in a Phase 3, double-blind, vehicle-controlled study of tapinarof cream for the treatment of atopic dermatitis (AD) in adults and children. The Phase 3 clinical program will enroll up to 800 patients across two pivotal trials (ADORING 1 and ADORING 2) to evaluate the safety and efficacy of tapinarof cream, 1% dosed once daily (QD) for 8 weeks versus vehicle cream QD in patients aged 2 years and older diagnosed with moderate to severe AD. The company anticipates announcing topline results from ADORING 1 and ADORING 2 in 1H 2023.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


