- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
HSP90 inhibition novel mechanism for treating hidradenitis suppurativa: JAMA
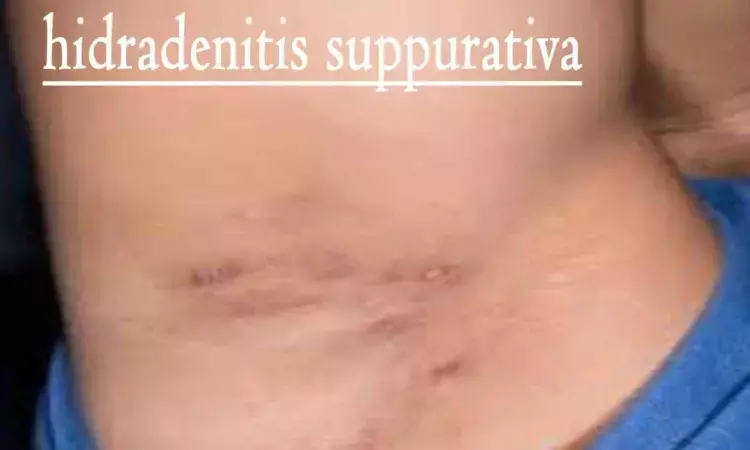
Denmark: Findings from a parallel-design, double-blind trial published in JAMA Dermatology have offered a potential new therapeutic option for treating hidradenitis suppurativa (HS). The study suggests the feasibility of HSP90 inhibition as a novel mechanism of action in HS treatment.
The trial of 15 patients showed that heat shock protein (HSP) 90 inhibition by oral RGRN-305, 250 mg, once daily led to a robust treatment response (n=10) versus placebo (n=5) after 16 weeks of treatment. Treatment-emergent adverse events (TEAEs) were not serious and were similarly frequent between the two groups (RGRN-305 and placebo).
Hidradenitis suppurativa is an immune-mediated, chronic, inflammatory skin disease that profoundly negatively influences the patient's quality of life, affecting approximately 1% of the population. It has limited treatment options, hence, there is a need for new treatments.
Hakim Ben Abdallah, Department of Dermatology and Venereology, Aarhus University Hospital, Aarhus, Denmark, and colleagues aimed to evaluate the feasibility of HSP 90 inhibition by RGRN-305 as a novel mechanism of action for the treatment of moderate to severe hidradenitis suppurativa.
The study included a 1- to 30-day screening period, a 16-week treatment period, and a 4-week follow-up period. It recruited individuals aged 18 years or older and moderate to severe hidradenitis suppurativa with six or more abscesses or inflammatory nodules in at least two distinct anatomic regions. Of the 19 patients screened, the study enrolled 15 patients (67% female; median age, 29 years). An intention-to-treat analysis was performed.
Patients were randomly assigned in a ratio of 2:1 to receive oral RGRN-305, 250-mg tablet, or matching placebo once daily for 16 weeks. The primary efficacy endpoint was the percentage of patients achieving Hidradenitis Suppurativa Clinical Response 50 (HiSCR-50) at week 16.
The study led to the following findings:
- The primary endpoint HiSCR-50 at week 16 was achieved by a higher percentage in the RGRN-305 group (60%) than in the placebo group (20%).
- Improvements were also observed across all secondary endpoints at week 16, including higher rates of the harder-to-reach HiSCR levels; 50% achieved HiSCR-75 and 30% achieved HiSCR-90, whereas none of the placebo-treated patients achieved HiSCR-75 or HiSCR-90.
- RGRN-305 was well tolerated, with no serious adverse events or deaths, and treatment-emergent adverse events were similarly frequent between the RGRN-305 and placebo groups.
"The findings of the randomized clinical trial suggest HSP90 inhibition by RGRN-305 to be a novel mechanism of action and a novel drug for HS treatment, providing good short-term safety and the potential to reduce the disease activity," the researchers wrote.
"These data warrant further clinical evaluations in larger trials to confirm the safety and efficacy of the drug, and also to explore this novel mode of action in other immune-mediated skin disorders," they concluded.
Reference:
Ben Abdallah H, Bregnhøj A, Emmanuel T, Ghatnekar G, Johansen C, Iversen L. Efficacy and Safety of the Heat Shock Protein 90 Inhibitor RGRN-305 in Hidradenitis Suppurativa: A Parallel-Design Double-Blind Trial. JAMA Dermatol. Published online December 06, 2023. doi:10.1001/jamadermatol.2023.4800
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


