- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Intravenous Immunoglobulin Demonstrates Efficacy in Treating Cutaneous Symptoms of Dermatomyositis
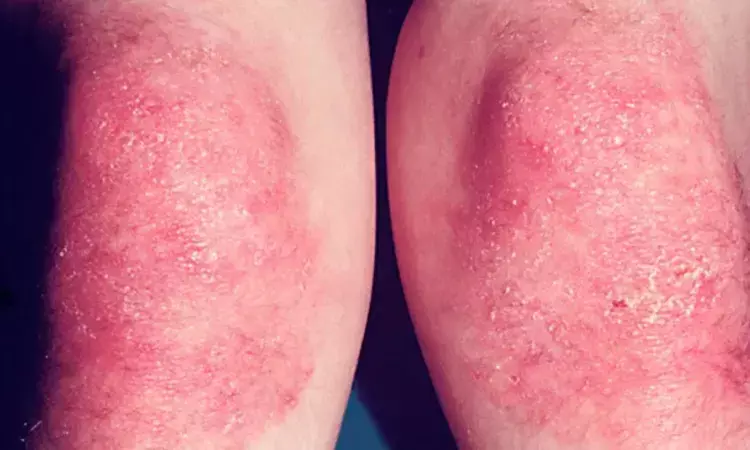
A recent Phase 3 clinical trial known as ProDERM has provided robust evidence supporting the use of intravenous immunoglobulin (IVIg) in treating the cutaneous symptoms of dermatomyositis (DM). Dermatomyositis is a rare autoimmune disease characterized by skin involvement. This study was published in The Lancet by Victoria P. Werth and colleagues.
In this double-blind, randomized, multicenter trial, adult patients with active DM were administered 2.0 g/kg of IVIg (Octagam 10%; Octapharma AG) or a placebo every 4 weeks during the first period of the study (Weeks 0–16). The subsequent open-label extension period (Weeks 16–40) involved all patients receiving IVIg for an additional 6 cycles. The efficacy of IVIg was assessed using various measures, including the modified cutaneous DM disease area and severity index activity (CDASI-A) and damage (CDASI-D) scores, as well as the myositis disease activity assessment tool (MDAAT), which includes a visual analogue scale (VAS).
- The study, conducted from February 2017 to November 2019, enrolled 95 patients who were randomly assigned to receive either IVIg (47 patients) or a placebo (48 patients) during the initial period.
- In total, 664 IVIg infusion cycles were administered, with a median dose of 2.0 g/kg. At Week 16, patients in the IVIg group showed a substantial mean CDASI-A change from baseline of -9.36 (95% CI: -12.52, -6.19), whereas the placebo group demonstrated a change of -1.16 (-3.32, 0.99), resulting in a highly significant difference (p < 0.0001).
- Similar improvements were observed in CDASI-D and the VAS of MDAAT. These positive outcomes were consistent across patients with varying degrees of disease severity at baseline.
ProDERM is the first large, prospective, and randomized trial to provide compelling evidence of IVIg's efficacy in improving the cutaneous manifestations of DM. IVIg treatment led to significant enhancements in dermatological symptoms, irrespective of the severity of the disease before treatment initiation. The study underscores the potential of IVIg as an effective therapeutic option for even the most severe cases of cutaneous DM. These findings represent a critical advancement in the management of DM, particularly its dermatological aspects, and may pave the way for improved treatment strategies for this challenging autoimmune condition.
Reference:
Werth, V. P., Aggarwal, R., Charles-Schoeman, C., Schessl, J., Levine, T., Kopasz, N., Worm, M., & Bata-Csörgő, Z. Efficacy of intravenous immunoglobulins (IVIg) in improving skin symptoms in patients with dermatomyositis: a post-hoc analysis of the ProDERM study. EClinicalMedicine,2023;64(102234):102234. https://doi.org/10.1016/j.eclinm.2023.102234
Dr Riya Dave has completed dentistry from Gujarat University in 2022. She is a dentist and accomplished medical and scientific writer known for her commitment to bridging the gap between clinical expertise and accessible healthcare information. She has been actively involved in writing blogs related to health and wellness.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


