- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Ixekizumab safe to use in Pediatric Patients with Moderate to Severe Plaque Psoriasis: JAMA
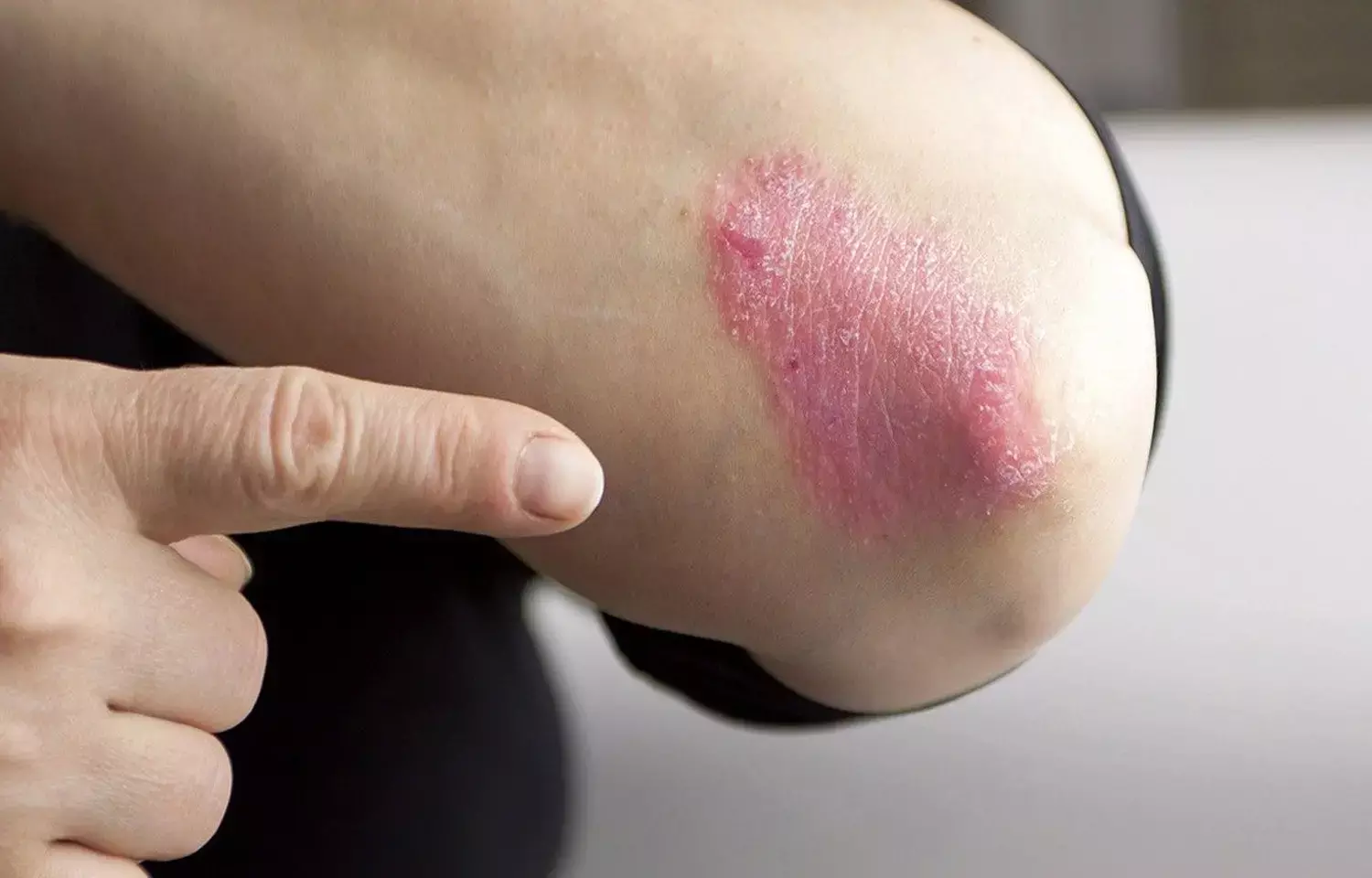
A new study conducted by Amy S. Paller and team showed improvements in patient-reported outcomes and objective measures of full psoriasis skin clearance among pediatric patients who took ixekizumab, and these response rates were sustained through week 108 of the trial.
The findings of this study were published in the Journal of American Medical Association.
Plaque psoriasis affects around 1% of children and adolescents globally. As a result, the purpose of this research was to assess the long-term effectiveness and safety of ixekizumab in pediatric treatment of moderate to severe psoriasis.
The IXORA-PEDS multinational randomized clinical study looked at pediatric patients with plaque psoriasis. Participants were 6 to 18 years old; had moderate to severe psoriasis; were applicants for phototherapy or systemic therapy; or had psoriasis that was not kept in check by topical therapies. From May to October 2021, data was analyzed using the intention-to-treat principle. The pediatric patients were randomly assigned to either a weight-based dosage of ixekizumab every four weeks or a placebo. After a 12-week placebo-controlled period, patients started a 48-week open-label ixekizumab management term (weeks 12-60), followed by a 108-week extension period. A sub-study looked at the randomized discontinuation of ixekizumab beyond week 60.
The key findings of this study were as follow:
1. A total of 171 patients were randomly assigned to receive either ixekizumab (n = 115) or a placebo (n = 56).
2. 139 (83.7%) of the 166 participants who started the maintenance stage completed week 108 of the experiment.
3. Patients achieved PASI 75, PASI 90, PASI 100, sPGA 0 or 1, and sPGA 0.
4. Primary and gated secondary end goals were maintained through week 108, with patients reaching PASI 75, PASI 90, PASI 100, sPGA 0 or 1, and sPGA 0.
5. Fifty-five patients (78.5%) reported an Itch Numeric Rating Scale improvement of four points or greater.
6. At week 108, elimination of nail psoriasis was reported in 68.1% (n = 28), palmoplantar psoriasis was reported in 90.0% (n = 10), scalp psoriasis was recorded in 76.2% (n = 83), and genital psoriasis was reported in 87.5% (n = 24) of patients who took ixekizumab.
7. During weeks 48 to 108 of the trial, there were no new safety findings, including no new occurrences of inflammatory bowel disease or candida infection.
In conclusion, the safety of ixekizumab was similar to previous reported findings in this cohort and the treatment's established safety profile.
Reference:
Paller AS, Seyger MMB, Magariños GA, et al. Long-term Efficacy and Safety of Up to 108 Weeks of Ixekizumab in Pediatric Patients With Moderate to Severe Plaque Psoriasis: The IXORA-PEDS Randomized Clinical Trial. JAMA Dermatol. Published online April 13, 2022. doi:10.1001/jamadermatol.2022.0655
Medical Dialogues consists of a team of passionate medical/scientific writers, led by doctors and healthcare researchers. Our team efforts to bring you updated and timely news about the important happenings of the medical and healthcare sector. Our editorial team can be reached at editorial@medicaldialogues.in.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


