- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
JAMA Study Finds Deucravacitinib Effective and Safe for Psoriasis Patients
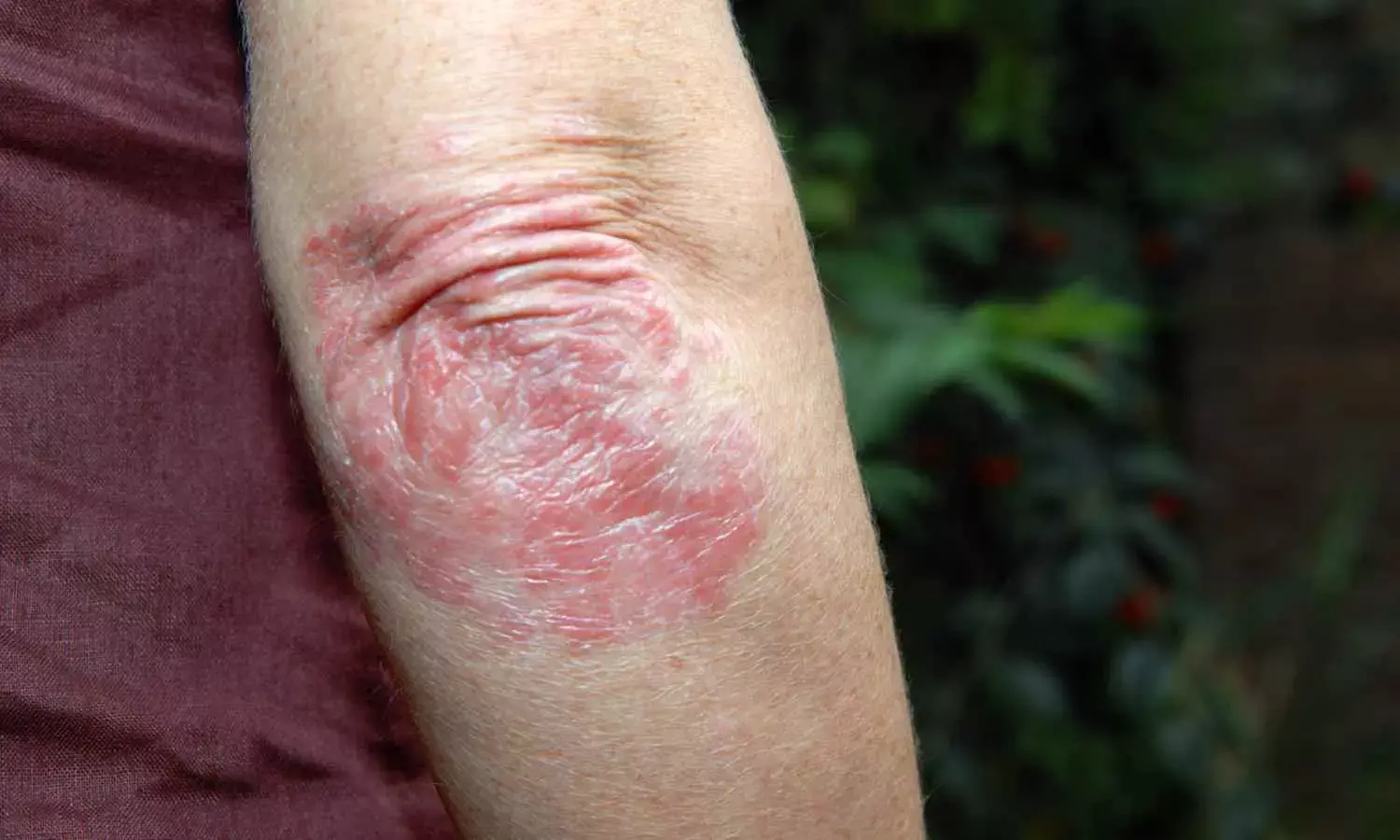
This recent 3-year study revealed that deucravacitinib which is an oral treatment for moderate to severe plaque psoriasis to offer a sustainable and safe long-term option for patients. The analysis combined data from the POETYK PSO-1 and PSO-2 randomized trials and a subsequent long-term extension (LTE) trial. This research published in the JAMA Dermatology evaluated the safety and efficacy of the drug through 148 weeks, despite challenges like the COVID-19 pandemic coinciding with the LTE trial period.
The PSO-1 and PSO-2 studies were global, double-blinded, phase 3 trials involving patients randomized to receive placebo, deucravacitinib, or apremilast. After 52 weeks, eligible patients could enroll in the nonrandomized LTE trial for open-label deucravacitinib therapy. From August 12, 2019, to June 15, 2022, 513 patients who began deucravacitinib treatment at day 1 transitioned to the LTE trial.
The cumulative analysis demonstrated that the safety profile of this treatment improved or remained consistent over time. Adverse event (AE) rates decreased from the first year (229.2 per 100 person-years) to the 3-year period (144.8 per 100 person-years). Serious AEs and discontinuations due to AEs also remained low, with slight reductions observed over time.
Common AEs, such as nasopharyngitis and upper respiratory tract infections, were less frequent during the three-year period. COVID-19 incidence increased during the pandemic but was well-managed. Other conditions of interest, including herpes zoster, cardiovascular events, and malignancies, occurred at low rates throughout the study.
Efficacy analysis revealed that patients maintained significant improvements in their psoriasis symptoms through 3 years. These measures included achieving a 75% or 90% reduction in Psoriasis Area and Severity Index (PASI 75/90) and reaching clear or almost clear skin ratings on the static Physician Global Assessment scale (sPGA 0/1). These results highlight the durable clinical benefits of continuous deucravacitinib therapy.
Overall, this study establishes deucravacitinib as a safe and effective long-term treatment for moderate to severe plaque psoriasis. By maintaining its efficacy and demonstrating a manageable safety profile over 3 years, deucravacitinib offers hope for sustained relief for patients with this chronic condition.
Source:
Armstrong, A. W., Lebwohl, M., Warren, R. B., Sofen, H., Imafuku, S., Ohtsuki, M., Spelman, L., Passeron, T., Papp, K. A., Kisa, R. M., Vaile, J., Berger, V., Vritzali, E., Hoyt, K., Colombo, M. J., Scotto, J., Banerjee, S., Strober, B., Thaçi, D., & Blauvelt, A. (2024). Safety and Efficacy of Deucravacitinib in Moderate to Severe Plaque Psoriasis For Up to 3 Years. In JAMA Dermatology. American Medical Association (AMA). https://doi.org/10.1001/jamadermatol.2024.4688
Neuroscience Masters graduate
Jacinthlyn Sylvia, a Neuroscience Master's graduate from Chennai has worked extensively in deciphering the neurobiology of cognition and motor control in aging. She also has spread-out exposure to Neurosurgery from her Bachelor’s. She is currently involved in active Neuro-Oncology research. She is an upcoming neuroscientist with a fiery passion for writing. Her news cover at Medical Dialogues feature recent discoveries and updates from the healthcare and biomedical research fields. She can be reached at editorial@medicaldialogues.in
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


