- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Nemolizumab: New Effective Drug for Management of Atopic Dermatitis, Study Finds
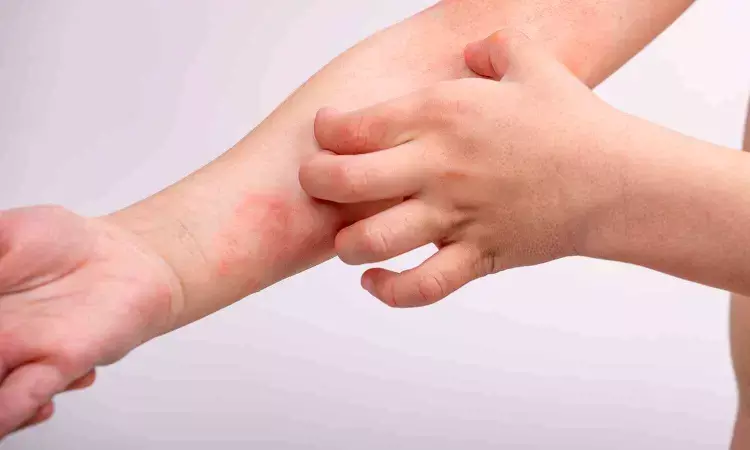
USA: Nemolizumab, a newly developed medication for atopic dermatitis, is effective when combined with topical corticosteroids (TCS) or topical calcineurin inhibitors (TCI), a recent randomized control trial has shown.
The trial report published in The Lancet Journal, suggests Nemolizumab when used in combination with TCS and TCI has shown to be effective and there have been significant clinical improvements in reducing inflammation and itch in both adults and adolescents with moderate-to-severe atopic dermatitis.
Atopic dermatitis is a chronic, itchy inflammatory skin disease commonly affecting children resulting in significant morbidity and greatly affecting the quality of life. Nemolizumab, an interleukin (IL)-31 receptor inhibitor has recently been introduced to manage atopic dermatitis, and a phase 3 clinical trial was conducted to assess its efficacy and safety.
For this purpose, Jonathan I Silverberg, Department of Dermatology, George Washington University School of Medicine and Health Sciences, Washington, DC, USA, and colleagues organized ARCADIA 1 and ARCADIA 2, a double-blind, placebo-controlled phase 3 trials in adult and adolescent participants, aged ≥12 years with moderate-to-severe atopic dermatitis, associated pruritus, and inadequate response to topical steroids.
Participants from 281 clinics, hospitals, and academic centers in 22 countries were enrolled in both trials. They were randomly assigned in a 2:1 ratio to receive either nemolizumab 30 mg subcutaneously (with a baseline loading dose of 60 mg) or a matching placebo every 4 weeks. This treatment was given alongside background topical corticosteroids (TCS) with or without topical calcineurin inhibitors (TCI).
Both study staff and participants were blinded throughout the study, with outcome assessors remaining blinded until the database was locked. Coprimary endpoints at week 16 post-baseline were Investigator's Global Assessment (IGA) success (score of 0 [clear skin] or 1 [almost clear skin] with a ≥2-point improvement from baseline) and at least 75% improvement in Eczema Area and Severity Index score from baseline (EASI-75 response).
Outcome rates were compared between groups with the Cochran–Mantel–Haenszel test adjusting for randomisation strata.
Efficacy analyses were done on an intention-to-treat basis; safety analyses included all participants who received one dose of nemolizumab or placebo.
The study reveals the following findings:
- Both trials achieved the co-primary endpoints. At week 16, a higher proportion of participants receiving nemolizumab plus TCS–TCI achieved IGA success compared to those receiving placebo plus TCS–TCI (ARCADIA 1: 221 [36%] of 620 vs 79 [25%] of 321, with an adjusted percentage difference of 11.5%, ARCADIA 2: 197 [38%] of 522 vs 69 [26%] of 265, adjusted difference 12·2%)
- EASI-75 response (ARCADIA 1: 270 [44%] vs 93 [29%], adjusted difference 14·9%, ARCADIA 2: 220 [42%] vs 80 [30%], adjusted difference 12·5%)
- Nemolizumab demonstrated significant benefits across all key secondary endpoints, including itch improvement as early as week 1 and sleep improvement by week 16.
- The safety profile was similar between nemolizumab plus TCS–TCI and placebo plus TCS–TCI.
- 306 participants in ARCADIA 1 and 215 participants ARCADIA 2 who received nemolizumab plus TCS–TCI had at least one treatment-emergent adverse event.
- 146 participants in ARCADIA 1 and 117 participants ARCADIA 2 who received placebo plus TCS–TCI had at least one treatment-emergent adverse event.
- In ARCADIA 2, ten serious treatment-emergent adverse events potentially related to nemolizumab were reported in five (1%) participants. No deaths were reported.
“Nemolizumab demonstrates improvements in inflammation and itch in adults and adolescents with moderate-to-severe atopic dermatitis. If approved, nemolizumab could provide a valuable addition to existing therapies,” said the researchers.
Reference: Silverberg JI, Wollenberg A, Reich A, Thaçi D, Legat FJ, Papp KA, Stein Gold L, Bouaziz JD, Pink AE, Carrascosa JM, Rewerska B, Szepietowski JC, Krasowska D, Havlíčková B, Kalowska M, Magnolo N, Pauser S, Nami N, Sauder MB, Jain V, Padlewska K, Cheong SY, Fleuranceau Morel P, Ulianov L, Piketty C; ARCADIA 1 and ARCADIA 2 Study Investigators. Nemolizumab with concomitant topical therapy in adolescents and adults with moderate-to-severe atopic dermatitis (ARCADIA 1 and ARCADIA 2): results from two replicate, double-blind, randomised controlled phase 3 trials. Lancet. 2024 Aug 3;404(10451):445-460. doi: 10.1016/S0140-6736(24)01203-0. Epub 2024 Jul 24. PMID: 39067461.
BDS, MDS(orthodontics)
Dr. Garima Soni holds a BDS (Bachelor of Dental Surgery) from Government Dental College, Raipur, Chhattisgarh, and an MDS (Master of Dental Surgery) specializing in Orthodontics and Dentofacial Orthopedics from Maitri College of Dentistry and Research Centre. At medical dialogues she focuses on dental news and dental and medical fact checks against medical/dental mis/disinformation
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


