- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Prophylactic monthly Garadacimab safe and effective against hereditary angioedema attacks
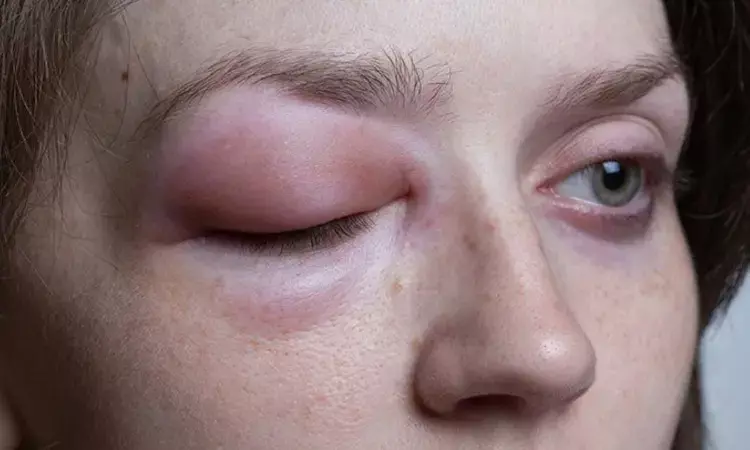
A new trial found that prophylactic subcutaneous administration of garadacimab every month in adults and adolescents was safe and has significantly decreased the attacks of hereditary angioedema compared to placebo. The trial results were published in the journal The Lancet.
Dysregulation of the kallikrein–kinin system leads to a rare and potentially life-threatening genetic disease called Hereditary angioedema. Recently Garadacimab (CSL312) which is a novel, fully human monoclonal antibody, is being studied for the prevention of hereditary angioedema attacks as it inhibits activated factor XII (FXIIa). VANGUARD is a pivotal, multicenter, randomized, double-blind, placebo-controlled, phase 3 trial that evaluated the efficacy and safety of once-monthly subcutaneous administrations of garadacimab as prophylaxis for hereditary angioedema.
VANGUARD trial recruited eligible patients aged ≥12 years with type I or type II hereditary angioedema across seven countries (Canada, Germany, Hungary, Israel, Japan, the Netherlands, and the USA). Using an interactive response technology (IRT) system, eligible patients were assigned randomly to garadacimab or placebo for 6 months (182 days) stratified by age (≤17 years vs >17 years) and baseline attack rate (1 to <3 attacks per month vs ≥3 attacks per month) for the adult group. All the participants and investigators were masked in a double-blind fashion. A loading dose of 400mg subcutaneous garadacimab was given as two 200-mg injections or volume-matched placebo on day 1 of the treatment period, followed by five additional self-administered (or caregiver-administered) monthly doses of 200 mg subcutaneous garadacimab or volume-matched placebo. Investigator-assessed time-normalized number of hereditary angioedema attacks was the primary endpoint during the 6-month treatment period (day 1 to day 182). Safety evaluation was done in patients who received at least one dose of garadacimab or placebo.
Key findings:
- Out of 80 patients who were screened, 65 eligible patients were with type I or type II hereditary angioedema.
- Of these 39 were randomly assigned to garadacimab and 25 to placebo.
- 38 (59%) of 64 participants were female and 26 (41%) were male.
- 55 (86%) of 64 participants were White, six (9%) were Asian (Japanese), one (2%) was Black or African American, one (2%) was Native Hawaiian or Other Pacific Islander, and one (2%) was listed as other.
- There was a significant decrease in the mean number of investigator-confirmed hereditary angioedema attacks per month in the garadacimab group (0·27, 95% CI 0·05 to 0·49) than in the placebo group (2·01, 1·44 to 2·57; p<0·0001) during the 6-month treatment period, corresponding to a percentage difference in means of –87% (95% CI –96 to –58; p<0·0001).
- The median number of hereditary angioedema attacks per month was 0 (IQR 0·00–0·31) for garadacimab and 1·35 (1·00–3·20) for placebo.
- Upper-respiratory tract infections, nasopharyngitis, and headaches were the most common treatment-emergent adverse events.
- There was no increased risk of bleeding or thromboembolic events with FXIIa inhibition.
Thus, monthly subcutaneous administration of garadacimab was found to be safe and effective as prophylactic therapy for adults and adolescents as it significantly reduced hereditary angioedema attacks in patients.
Further reading: Efficacy and safety of garadacimab, a factor XIIa inhibitor for hereditary angioedema prevention (VANGUARD): a global, multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. https://doi.org/10.1016/S0140-6736(23)00350-1
BDS, MDS
Dr.Niharika Harsha B (BDS,MDS) completed her BDS from Govt Dental College, Hyderabad and MDS from Dr.NTR University of health sciences(Now Kaloji Rao University). She has 4 years of private dental practice and worked for 2 years as Consultant Oral Radiologist at a Dental Imaging Centre in Hyderabad. She worked as Research Assistant and scientific writer in the development of Oral Anti cancer screening device with her seniors. She has a deep intriguing wish in writing highly engaging, captivating and informative medical content for a wider audience. She can be contacted at editorial@medicaldialogues.in.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


