- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Remibrutinib improves outcomes in Chronic Spontaneous Urticaria Patients
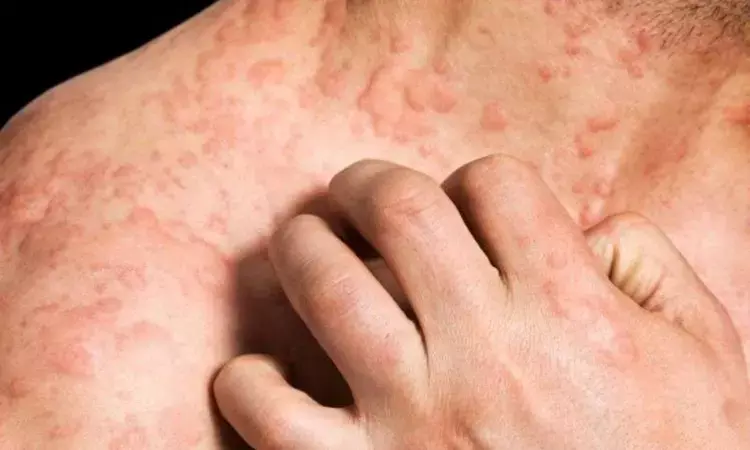
Patients with chronic spontaneous urticaria (CSU) who have found little relief with existing treatments, a novel drug called Remibrutinib (LOU064) has shown fast and sustained disease control, says the study in Journal of Allergy and Clinical Immunology. Remibrutinib, an oral, highly selective Bruton's tyrosine kinase inhibitor (BTKi), is currently in phase 3 development for CSU, offering hope to those who remain symptomatic despite second-generation H1-antihistamines.
In a phase 2b extension study, patients who previously participated in the core study and still had a weekly Urticaria Activity Score (UAS7) of 16 or higher received Remibrutinib at a dose of 100 mg twice daily for 52 weeks. The primary goal was to evaluate the long-term safety and tolerability of the drug. Key efficacy measures included changes in UAS7 and the proportion of patients achieving a complete response (UAS7=0) and well-controlled disease (UAS7≤6) at week 4 and over the 52-week period.
Out of 230 patients, 194 entered the treatment phase, receiving at least one dose of Remibrutinib. The safety profile of Remibrutinib remained consistent with that observed in the core study. Most treatment-related adverse events were mild to moderate and not linked to the drug. The most common adverse events involved infections (30.9%), skin and subcutaneous issues (26.8%), and gastrointestinal problems (16.5%).
At both week 4 and 52, there was a significant reduction in UAS7 from baseline, with a mean change of -17.6±13.40 at week 4 and -21.8±10.70 at week 52. Furthermore, 28.2% of patients achieved a UAS7 of 0 at week 4, rising to 55.8% at week 52. Notably, 52.7% of patients had their disease well-controlled (UAS7≤6) at week 4, increasing to 68.0% at week 52.
The results indicate that Remibrutinib offers a consistent safety profile and fast, sustained efficacy for up to 52 weeks in CSU patients inadequately controlled with H1-antihistamines. Further research and clinical trials will be necessary to confirm these promising findings and potentially provide a breakthrough treatment option for those with chronic spontaneous urticaria.
Reference:
Jain, V., Giménez-Arnau, A., Hayama, K., Reich, A., Carr, W., Tillinghast, J., Dahale, S., Lheritier, K., Walsh, P., Zharkov, A., Hugot, S., & Haemmerle, S. (2023). Remibrutinib demonstrates favorable safety profile and sustained efficacy in chronic spontaneous urticaria over 52 weeks. In Journal of Allergy and Clinical Immunology. Elsevier BV. https://doi.org/10.1016/j.jaci.2023.10.007
Neuroscience Masters graduate
Jacinthlyn Sylvia, a Neuroscience Master's graduate from Chennai has worked extensively in deciphering the neurobiology of cognition and motor control in aging. She also has spread-out exposure to Neurosurgery from her Bachelor’s. She is currently involved in active Neuro-Oncology research. She is an upcoming neuroscientist with a fiery passion for writing. Her news cover at Medical Dialogues feature recent discoveries and updates from the healthcare and biomedical research fields. She can be reached at editorial@medicaldialogues.in
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


