- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Risankizumab effective remedy for in pityriasis rubra pilaris: Study
Source- Brocco E, Laffitte E. Risankizumab for pityriasis rubra pilaris. Clin Exp Dermatol. 2021 Oct;46(7):1322-1324. doi: 10.1111/ced.14715. Epub 2021 Jun 13. PMID: 33914925.
Risankizumab effective in pityriasis rubra pilaris : CED
Pityriasis rubra pilaris (PRP) is a rare dermatological disease characterized by follicular hyperkeratotic papules and plaques. Till date there are no robust studies showing efficacy of drugs in this disease. As it shares similar inflammatory pathways and cytokine activation profile with psoriasis on-label psoriasis treatments have recently been used off-label to treat PRP. Risankizumab is an anti-interleukin (IL)-23p19 monoclonal IgG1 antibody used in treatment of psoriasis. Recently a case report describing efficacy of risankizumab in PRP was highlighted in Clinical and Experimental Dermatology journal.
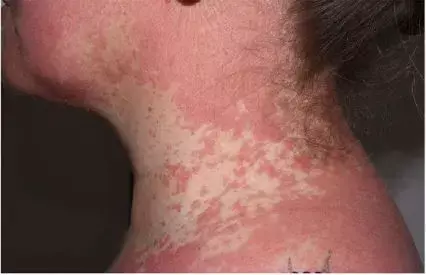
A 43-year-old woman presented with a 10-week history of a pruritic erythematous keratotic follicular papules progressing cephalocaudally, painful nail hyperkeratosis and palmoplantar keratoderma. The patient had a known history of untreated latent tuberculosis infection (LTBI). Histological examination showed hyperkeratosis with discrete, predominantly follicular spongiosis and acanthosis, along with the typical alternating orthokeratosis and parakeratosis (checkerboard pattern) with a dermal lymphocytic infiltrate. Based on the clinical and histopathological findings, PRP was diagnosed. The total body surface area (BSA)involvement was 84%. Physician Global Assessment (PGA) gave the maximum score of 5, while the Dermatology Life Quality Index (DLQI) score was 20 of 30.
Previous treatments with moisturizers, topical-class intravenous corticosteroids, ultraviolet therapy, antihistamines and two intramuscular cortisone injections remained ineffective. So the authors decided to treat the patient with risankizumab. For the untreated latent TB, 3 weeks prior to starting risankizumab, patient was treated with rifampicin 600 mg/day for 4 months. Risankizumab was started on the dosing regimen approved for psoriasis, with two injections of 75 mg administered subcutaneously at Weeks 0 and 4, followed by injections every 12 weeks. After the first Risankizumab injection, rapid clinical improvement was observed, with decrease in the affected BSA from 84% to 30%, along with reduction of DLQI from 20 to 13 and in PGA score from 5 to 2 (Fig. 1c,d). No major adverse effects (AEs) were reported. After 4 months of treatment, there was full cutaneous recovery, with no clinical relapse at the 12-month follow-up.
The classic treatments of PRP include oral isotretinoin, acitretin, methotrexate and anti-tumour necrosis factor-alpha drugs, which usually produce only scant to moderate improvement of the skin so newer case reports of use of biologicals to treat PRP have come in literature.
Upper respiratory infection, arthralgia and headache have been few common side effects reported with anti-IL-23p19 therapy. It is not associated with elevated risk of developing opportunistic infections, developing mucocutaneous Candida infection or inflammatory bowel disease. In addition in case of rizankizumab therapy for patients with LTBI, data shows no activation of TB for patients undergoing TB prophylaxis. Some data have showed absence of TB activation in subgroups of patients with LTBI treated with risankizumab even without TB prophylaxis.
In conclusion, the present case of PRP showing rapid and almost complete response within 4 weeks to risankizumab with no relapse after a year of follow-up makes it a promising therapy for patients with PRP and a safe options for patients with added risk of latent tuberculosis infection (LTBI).
Source- Brocco E, Laffitte E. Risankizumab for pityriasis rubra pilaris. Clin Exp Dermatol. 2021 Oct;46(7):1322-1324. doi: 10.1111/ced.14715. Epub 2021 Jun 13. PMID: 33914925.
MBBS
Dr Manoj Kumar Nayak has completed his M.B.B.S. from the prestigious institute Bangalore medical college and research institute, Bengaluru. He completed his M.D. Dermatology from AIIMS Rishikesh. He is actively involved in the field of dermatology with special interests in vitiligo, immunobullous disorders, psoriasis and procedural dermatology. His continued interest in academics and recent developments serves as an inspiration to work with medical dialogues.He can be contacted at editorial@medicaldialogues.in.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


