- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Roflumilast Cream 0.3% Safe and Effective for Plaque Psoriasis in Children Aged 2-11 Years: Study
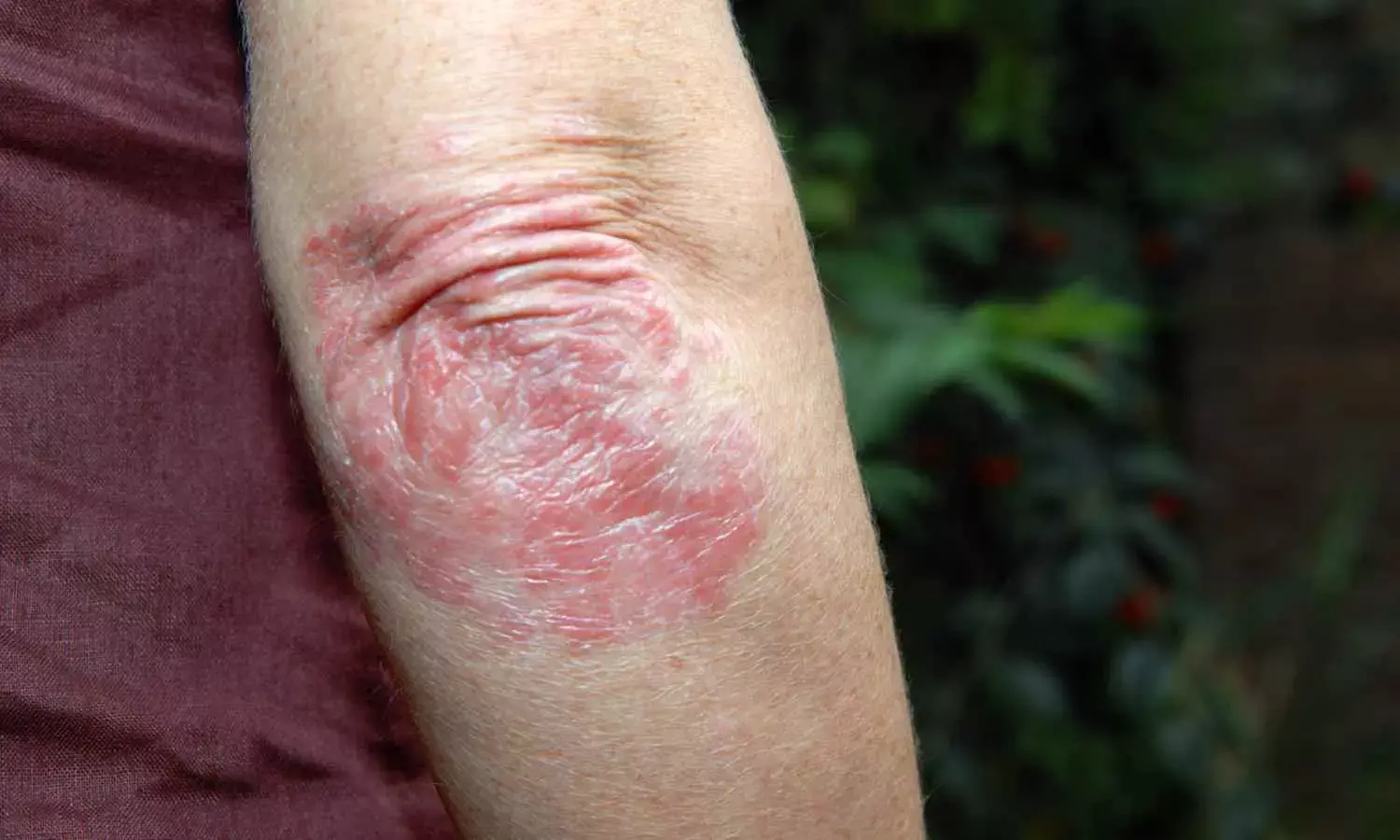
A new study published in the journal of Pediatric Dermatology found that under optimal usage settings, roflumilast cream 0.3% was safe, well-tolerated, and effective in reducing plaque psoriasis signs and symptoms in children ages 2 to 11.
A new nonsteroidal treatment with the potential to effectively manage illness and provide good safety is roflmilast cream 0.3%, a selective phosphodiesterase-4 (PDE4) inhibitor. When given once daily to children with mild to moderate plaque psoriasis aged 2-11 years, recent phase 2 trials have offered preliminary insights into its pharmacokinetics, therapeutic advantages, and tolerability.
Children with plaque psoriasis were the subjects of the trials that assessed roflumilast cream 0.3%. Trial 215 recruited patients aged 6–11 years (N=20; approvals June 26, 2020, and February 4, 2021), whereas Trial 216 was open to patients aged 2–5 years (N=10; protocol approvals August 24, 2020, and March 2, 2021).
The patients who were eligible had an Investigator Global Assessment (IGA) score of at least two and a body surface area (BSA) involvement of at least 2%. For 28 days, caregivers administered roflumilast once daily to the afflicted regions. All subjects underwent pharmacokinetic (PK) sample at Week 4, while maximum use subgroups (≥3% BSA involvement, excluding scalp, palms, and soles) underwent PK sampling at Week 2.
Predose plasma concentrations were used to derive extrapolated AUC0–24 values, which were then confirmed in a subset of patients by serial PK sampling. Tolerability, safety, and PK were the main endpoints. IGA success, PASI-75, itching reduction (WI-NRS), BSA change, and the Children's Dermatology Life Quality Index (CDLQI) were among the endpoints used to assess exploratory effectiveness.
In line with earlier research, the majority of patients showed signs of systemic exposure to roflumilast and its active N-oxide metabolite after daily use. Once-daily roflumilast cream 0.3% was well tolerated under maximal usage settings and improved psoriasis signs and symptoms in children ages 2–11, which is in line with phase 3 outcomes in adults and adolescents.
Overall, under optimal usage settings, roflumilast cream 0.3% was well tolerated and reduced psoriasis signs and symptoms in individuals ages 2–11. Overall PK, safety, tolerability, and effectiveness were in line with phase 3 results in patients aged ≥2 years and maximal use outcomes in patients aged ≥12 years, despite the small sample size.
Source:
Hebert, A. A., Guide, S. V., Groysman, V., Gonzalez, M. E., Blanco, D., Laquer, V., Seal, M. S., Thurston, A., Krupa, D., Snyder, S., Burnett, P., Chu, D. H., Berk, D. R., & Higham, R. C. (2025). Early evidence of safety, clinical benefit, and pharmacokinetics of roflumilast cream 0.3% once daily for treatment of mild or moderate plaque psoriasis in children aged 2–11 years. Pediatric Dermatology,. https://doi.org/10.1111/pde.70013
Neuroscience Masters graduate
Jacinthlyn Sylvia, a Neuroscience Master's graduate from Chennai has worked extensively in deciphering the neurobiology of cognition and motor control in aging. She also has spread-out exposure to Neurosurgery from her Bachelor’s. She is currently involved in active Neuro-Oncology research. She is an upcoming neuroscientist with a fiery passion for writing. Her news cover at Medical Dialogues feature recent discoveries and updates from the healthcare and biomedical research fields. She can be reached at editorial@medicaldialogues.in
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


