- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Roflumilast cream effective for treating plaque psoriasis: JAMA
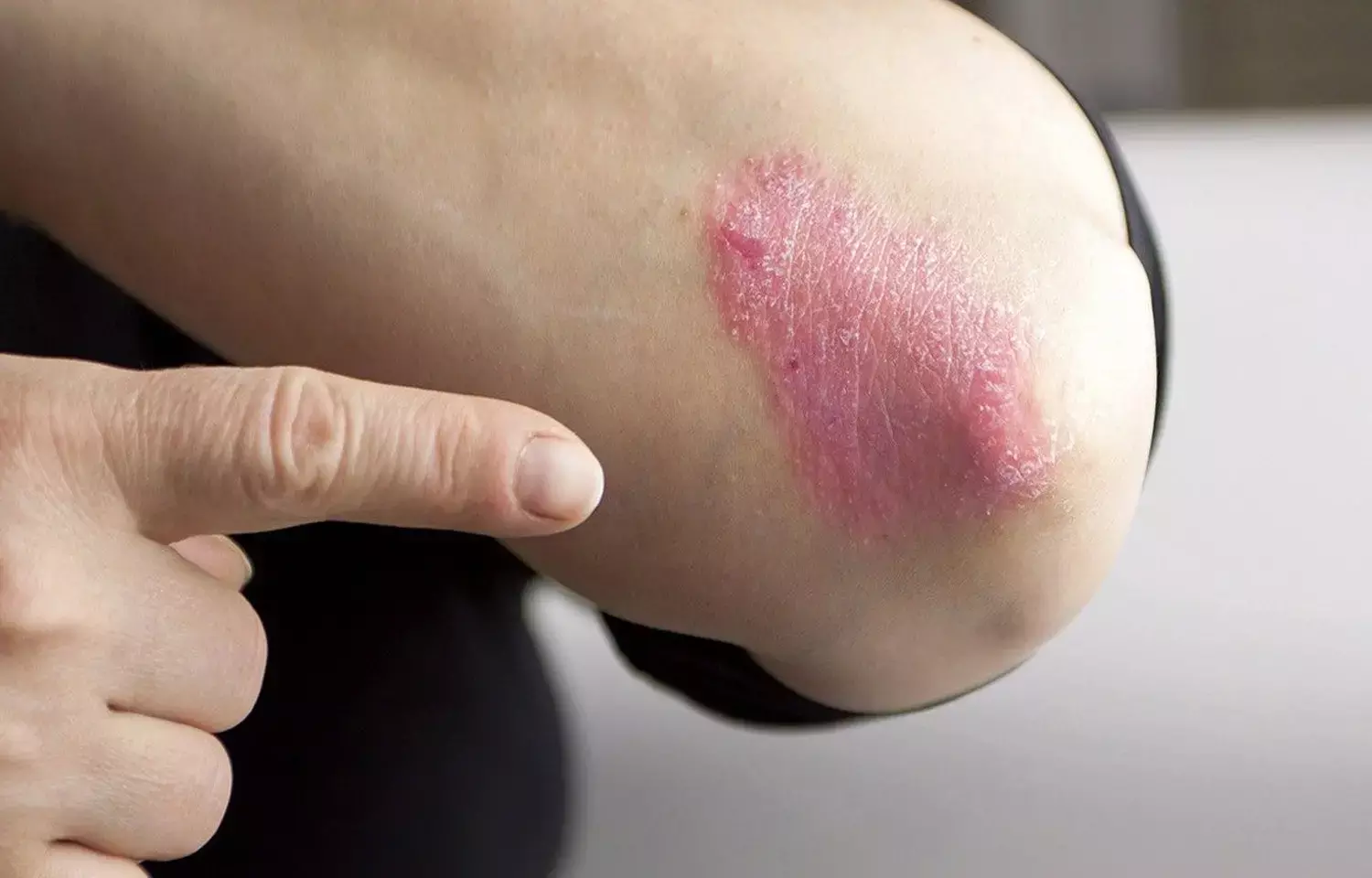
USA: According to a study published in JAMA Network, roflumilast cream was effective in treating plaque psoriasis in patients after 8 weeks of therapy.
A once-daily topical medication called roflumilast cream is used to treat psoriasis in both adults and teenagers. It contains a very effective, selective phosphodiesterase type 4 inhibitor (PDE4). Despite being well-known for its application in the treatment of chronic obstructive pulmonary disease, roflumilast was examined in two clinical studies for its efficacy in the topical management of plaque psoriasis. It functions as both a strong and selective phosphodiesterase 4 (PDE4) inhibitor.
The investigators aimed to assess the effectiveness of 0.3% roflumilast cream, used twice daily for 8 weeks on individuals with plaque psoriasis.
According to Mark G. Lebwohl, MD, Icahn School of Medicine at Mount Sinai, New York, and colleagues, treating psoriasis in intertriginous areas can be challenging because the skin there is thinner and more sensitive, allows for greater drug absorption, and is vulnerable to side effects from topical treatments.
The 2 clinical trials, DERMIS-1 and DERMIS-2, had a combined total of 881 recruited participants. They were parallel-group, double-blind, and vehicle-controlled studies. Age 2 years or older and a clinical plaque psoriasis diagnosis over the previous 6 months were prerequisites for inclusion into the research. The researchers required plaque psoriasis to impact between 2 and 20% of body surface area, excluding palms, scalp, and foot soles, in order for patients with psoriasis on the limbs, trunk, face, and/or intertriginous areas to be included in the study. Participation was only authorized if the Investigator Global Assessment (IGA) rating was at least mild.
The study team randomly assigned each eligible participant, 2 to 1, to either vehicle cream once daily or roflumilast cream, 0.3%. Individual therapies were unknown to everyone involved, including patients, healthcare personnel, investigators, and sponsors. The primary effectiveness endpoint was established by the investigators as patients receiving an IGA rating of clear or almost clear along with a 2-grade improvement over baseline by the end of the 8-week experiment. The intertriginous IGA rating success, the Worst Itch Numeric Rating Scale (WI-NRS) score, and a decrease in the Psoriasis Area and Severity Index (PASI) score were among the nine secondary outcomes that were measured.
Key highlights of the study:
- By 8 weeks, 108 participants (42.4%) who received roflumilast had reached the primary objective, compared to 8 patients (6.1%), who received the vehicle (difference, 39.6%; 95% CI, 32.3 - 46.9%; P =.001).
- In DERMIS-2, they reported that 99 patients (37.5%) treated with roflumilast and 9 persons (6.9%) in the vehicle arm both achieved the primary endpoint (difference 28.8%; 95% CI, 20.8 - 36.9%; P =.001).
- In both experimental treatment arms compared to control groups, the investigators saw statistically significant percentages of IGA success by 8 weeks.
- In both studies, intertriginous IGA success, PASI score, and WI-NRS success were among the 9 secondary endpoints where there were statistically significant differences favoring roflumilast compared with vehicle therapy.
- Roflumilast, when applied topically, had a lower risk of side effects than oral PDE4 inhibitors.
The outcomes of both trials point to promising results for the prospective use of the medication for plaque psoriasis, even though the researchers note that more research will be required to investigate long-term safety and efficacy in comparison to alternative treatments.
Given that topical medicines normally have a harder time treating psoriasis in these areas due to the skin being thinner and more sensitive, the researchers came to the conclusion that these data imply roflumilast may be more beneficial for intertriginous skin treatment.
REFERENCE
Lebwohl MG, Kircik LH, Moore AY, et al. Effect of Roflumilast Cream vs Vehicle Cream on Chronic Plaque Psoriasis: The DERMIS-1 and DERMIS-2 Randomized Clinical Trials. JAMA. 2022;328(11):1073–1084. doi:10.1001/jama.2022.15632
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


