- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Spesolimab Shows Promise for Generalized Pustular Psoriasis: Study
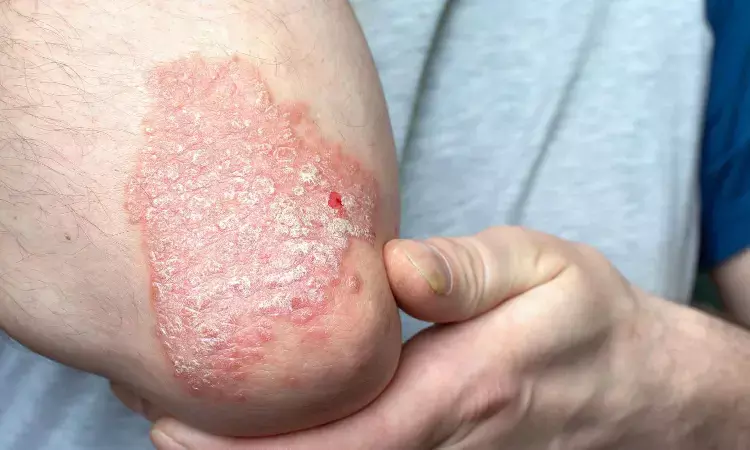
Post-hoc analyses of the EFFISAYIL 2 trial revealed improvements in Psoriasis Symptom Scale (PSS), Pain Visual Analog Scale (VAS), and Dermatology Life Quality Index (DLQI) scores. These findings suggest potential benefits of continuous subcutaneous spesolimab treatment for generalized pustular psoriasis (GPP).
Boehringer Ingelheim announced new analyses to be presented at the American Academy of Dermatology (AAD) annual meeting in Orlando that explore the effect that SPEVIGO® (spesolimab-sbzo) may have on the physical symptoms and mental health burden associated with generalized pustular psoriasis (GPP). These post-hoc exploratory analyses from the EFFISAYIL® 2 clinical trial assessed the efficacy and safety of subcutaneous SPEVIGO in patients suffering from GPP.
Distinct from plaque psoriasis, GPP is a serious and rare, chronic, heterogenous, inflammatory neutrophilic disease associated with painful skin manifestations and systemic symptoms, such as fever, pain, and fatigue. GPP’s unpredictable nature can potentially have significant long-term impacts on quality of life for people living with it and may cause fear and anxiety over the disease course.
“Generalized pustular psoriasis profoundly affects patients' physical and mental well-being, often disrupting their education, careers, and personal relationships. We are encouraged by the improvements observed in patient-reported outcomes with spesolimab. These studies strengthen our commitment to delivering innovative treatments for those living with severe dermatological conditions like GPP,” says Vicky Brown, Senior Vice President and Head of Immunology, Oncology, and Eye Health, Boehringer Ingelheim Pharmaceuticals, Inc.
In the EFFISAYIL 2 clinical trial, patients with a history of GPP were randomized to receive one of three active treatment regimens or a placebo. Participants were asked to rate their GPP symptoms – including pain, redness, itching, and burning – using the Psoriasis Symptom Scale (PSS), the Pain Visual Analog Scale (VAS), and the Dermatology Life Quality Index (DLQI). The PSS ranges from 0 to 16, while the Pain VAS ranges from 0 to 100, with higher scores indicating more severe symptoms. Of the 30 patients randomized to receive subcutaneous spesolimab, 300 mg every four weeks after a 600mg loading dose, participants reported a reduction in mean Pain VAS scores over the course of the trial, with more than half (56.5%) of patients reporting a Pain VAS score of 0 at Week 48 and the mean PSS score decreasing from 5.34 to 2.96 by Week 48.
Patients were also asked to rate the impact of GPP through a 10-item questionnaire to assess changes in quality of life using the DLQI. The results found that mean DLQI scores improved from a “very large effect on patient’s life” score (11.14) at baseline to a “small effect on patient’s life” score (4.57) at week 48 following treatment with SPEVIGO.
Measurable improvements in mean PSS, Pain VAS and DLQI scores were observed by the first assessment at Week 4.
“The results from EFFISAYIL 2 provide valuable insights into the chronicity of generalized pustular psoriasis,” said Dr. Tina Bhutani, study author. “The data are a pivotal step in advancing patient-centered care in GPP.”
These findings may provide healthcare professionals with data that can help inform their choice of treatment regimens for people living with GPP.
About SPEVIGO
SPEVIGO® is a novel, humanized, selective antibody that specifically blocks the activation of the IL-36R, a signaling pathway within the immune system shown to be involved in the pathogenesis of several autoinflammatory diseases, including GPP. It is the first targeted therapy for the treatment of GPP and has been evaluated in the largest clinical program specifically for the treatment of patients with GPP.
What is SPEVIGO?
SPEVIGO® is a prescription medicine used to treat generalized pustular psoriasis (GPP) in adults and children 12 years of age and older who weigh at least 88 pounds (40 kg). It is not known if SPEVIGO® is safe and effective in children under 12 years of age or who weigh less than 88 pounds (40kg).
About the EFFISAYIL clinical trial program
The EFFISAYIL® clinical trial program evaluated the largest and broadest population of GPP patients in trials of a therapy specifically targeting the IL-36 pathway for GPP:
- EFFISAYIL® 1: A Phase II study that demonstrated treatment with a single intravenous dose of spesolimab significantly improved signs and symptoms of GPP in patients experiencing a flare, including rapid pustular and skin clearance. These results supported the approval of spesolimab as the first specific treatment for GPP flares in adults in major markets
- EFFISAYIL® 2: A Phase IIb study that showed spesolimab significantly reduced the risk of GPP flares by 84% over 48 weeks compared to placebo. In the trial with 123 patients, no flares were observed after week 4 of spesolimab SC treatment in the high-dose group (n=30)
- EFFISAYIL® ON: An open-label extension study to evaluate the long-term safety and efficacy of spesolimab in patients with GPP who have completed previous spesolimab trials.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


